Interesting Case October 2023
Ifeoluwatomi Fuwape, M.D.
Pathology resident PGY3
Wei Chen, M.D.
Attending Gastrointestinal and hepatobiliary pathologist
Department of Pathology, Duke University Medical Center
Durham, North Carolina 27710
Case History
The patient is a 70-year-old male with a history of hepatitis C complicated by cirrhosis and hepatocellular carcinoma, status post orthotopic liver transplant (OLT) two years ago with duct-to-duct anastomosis and Cytomegalovirus (CMV) seromismatch (donor positive and recipient negative). At the transplant, nucleic acid amplification testing for Hepatitis C virus (HCV) was positive (6.7 LOG IU/ml). Epstein-Barr Virus (EBV) was negative in both donor and recipient.
Post-transplant he had multiple episodes of acute T-cell mediated rejection. He had diverticulitis status post partial colectomy, perirectal abscesses, and fistulas. He presented to the hospital with five days of worsening rectal pain and increased flatulence, loss of appetite, urgency, diarrhea, and weight loss. Laboratory studies revealed elevated Alkaline phosphatase (162 U/L), normal AST (25 U/L), normal ALT (23 U/L) and normal alpha fetoprotein (1.2 ng/mL). Magnetic resonance imaging (MRI) showed a new T2 intermediate, hypo enhancing caudate lobe lesion, 3 cm, concerning for malignancy. No lymphadenopathy is seen. A mass-targeted biopsy was performed.
Pathologic Findings
Histologic sections reveal biopsy with no hepatocytes or normal liver architecture seen. The biopsy tissue is composed of large atypical lymphoid cells in a prominent fibrotic background (Figure 1-2). There are occasional residual bile ducts with bland cytological features. No epithelial damage or associated inflammation is identified. Immunohistochemistry reveals the large, atypical cells are positive for CD30, CD20, and EBER (EBV) (Figure 3-5). The cells are also positive for MUM1 and BCL6, and Ki-67 stain highlights many of these large lymphoid cells.
Diagnosis
EBV positive, CD30 positive, B-cell post-transplant lymphoproliferative disorder (PTLD), destructive type, monomorphic variant of diffuse large B-cell lymphoma.
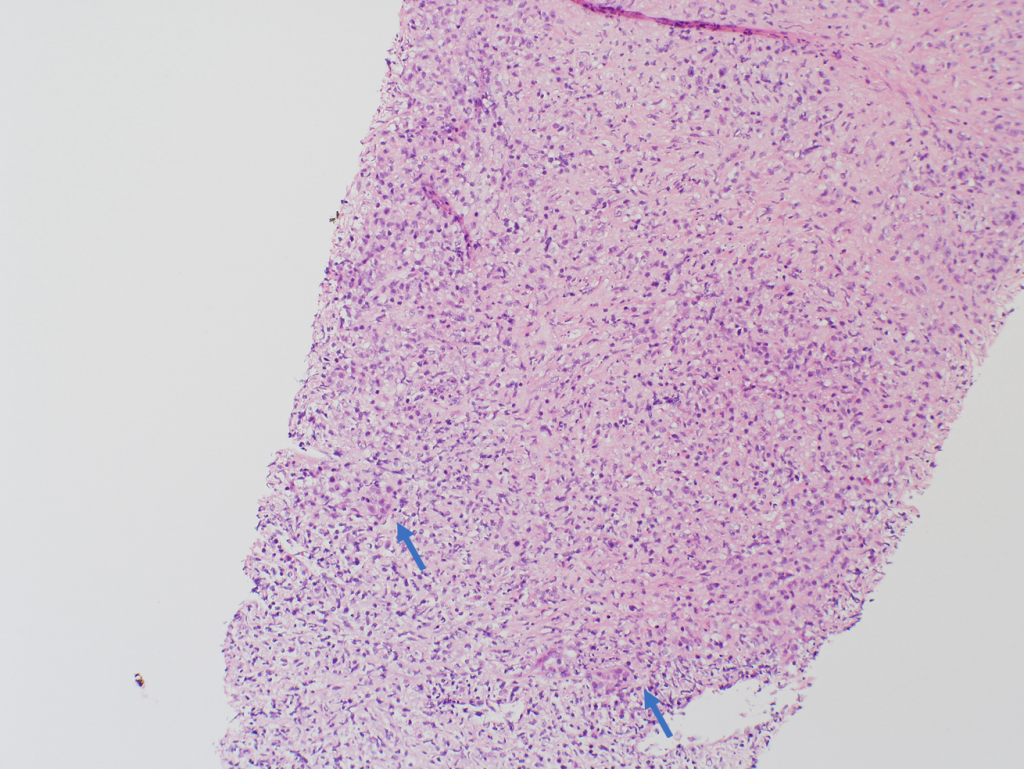
Figure 1. H&E stain. 100x. Atypical lymphoid cells in a fibrotic background. No residual hepatocytes are present. The arrows showing the residual bile ducts.
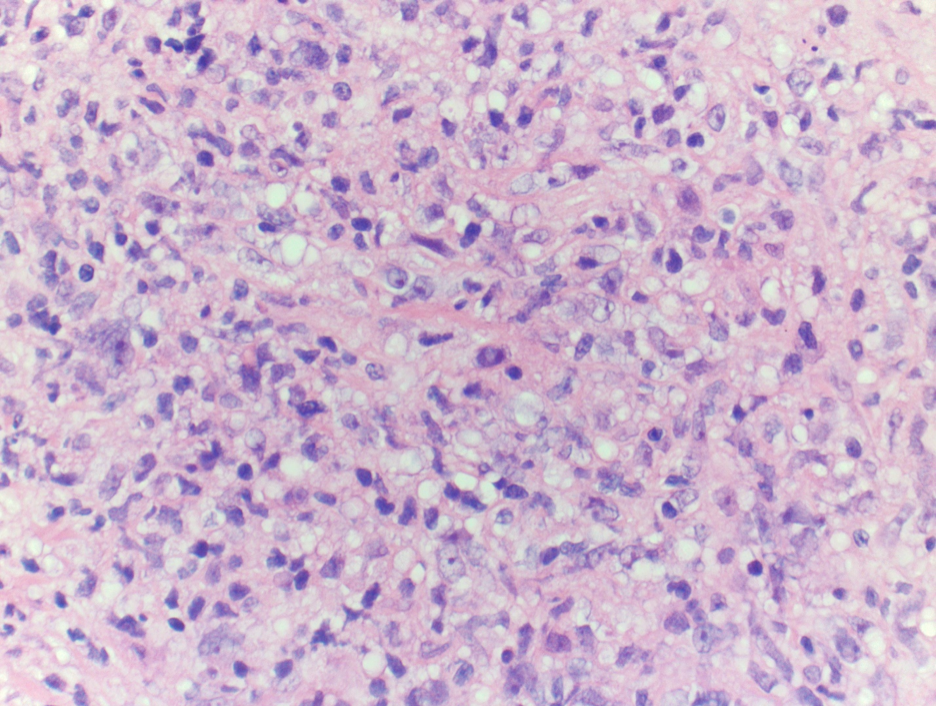
Figure 2. H&E stain. 400x. Atypical lymphoid cells in a fibrotic background
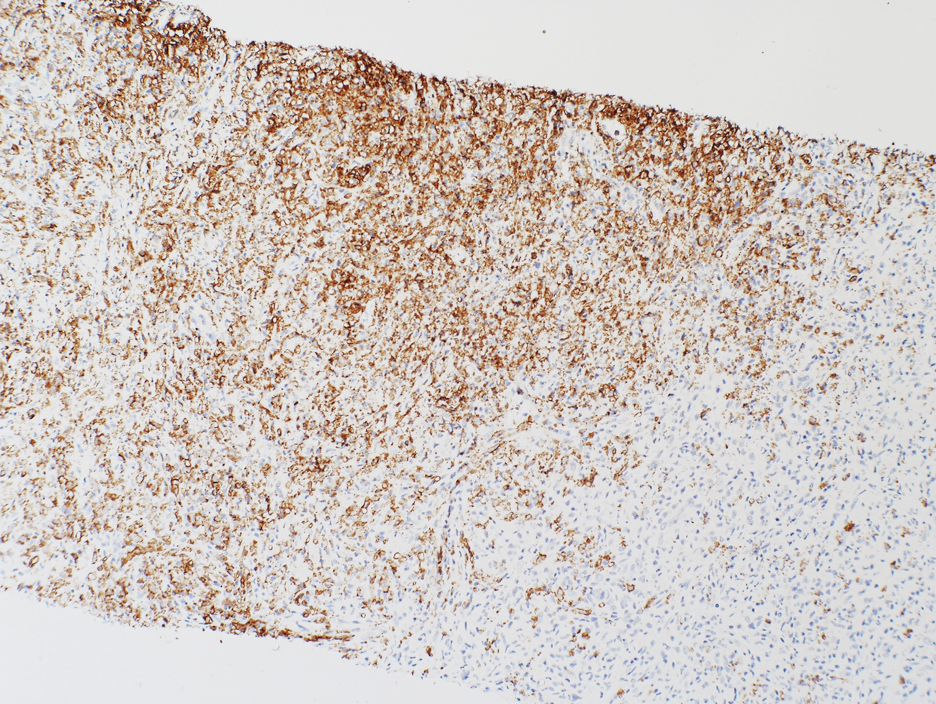
Figure 3. CD20 stain.100x.
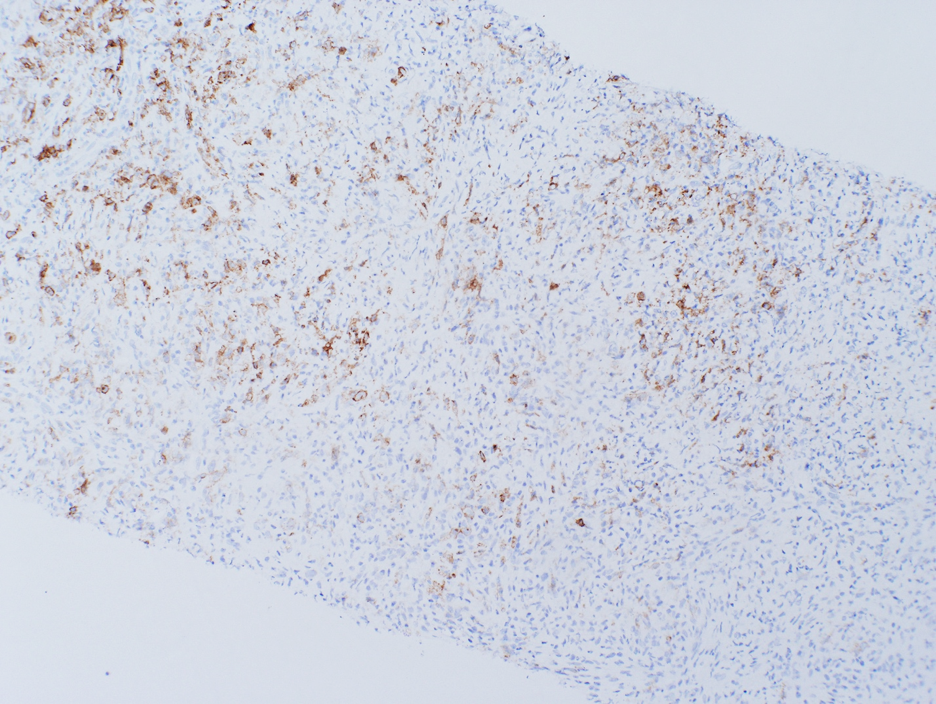
Figure 4. CD30 stain. 100x. Partially positive in the atypical cells.
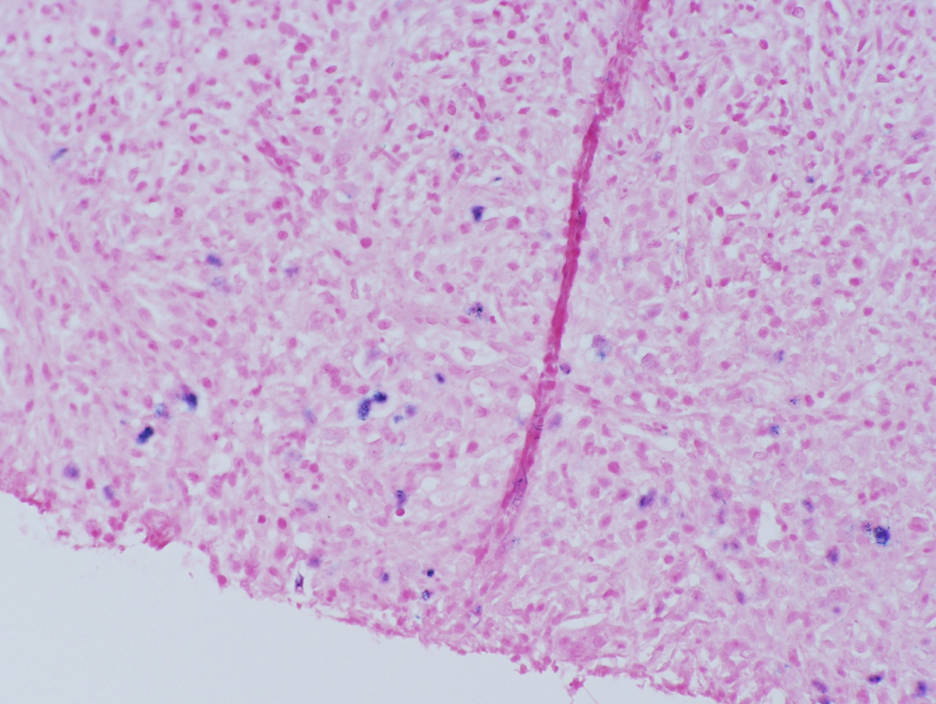
Figure 5. EBER CISH. 200x. Highlights some of the atypical cells.
Discussion
Post-transplant lymphoproliferative disorder (PTLD) is a lymphoplasmacytic disease that may occur after any type of organ transplant in the setting of immunosuppression. The reported incidence of PTLD in liver recipient is approximately 2.7%, which appears to be low compared to that of other solid organ transplants (1). However, the numbers vary among transplant centers.
Multiple risk factors are reported contributing to the development of PTLD, including the degree of immunosuppression, EBV status, time post-transplantation, and recipient factors such as age, type of allograft, underlying disease and other viral infections (2). Long-term immunosuppression is considered a major risk factor for the lymphoma. Studies in solid organ transplant demonstrated that the degree of immunosuppression, particularly among pediatric patients and those exposed to certain types of induction therapy or prolonged high doses of tacrolimus was closely associated with increasing incidence of PTLD (3, 4). EBV infection is another important etiology for the disease. The reported presence of EBV DNA within neoplastic tissue supported a role of viral proteins in the propagation of PTLD. In a study of 276 pediatric kidney transplant recipients, patients with EBV seromismatch (i.e., a negative recipient and a positive donor) suffered a 7.7-fold increased risk compared to the control group (hazard ratio [HR] 7.7, 95% CI 1.6-35.9)(4). However, EBV-negative tumors can also occur. In addition, pediatric population (age 0 to 18 years) was reported to have increased PTLD incidence up to 15% after liver transplant (5).
More than half of PTLD presents with extranodal masses. Involved organs include the gastrointestinal tract (stomach, intestine), lungs, skin, liver, central nervous system, and the allograft itself. In our case, the finding of an allograft liver mass in a patient with history of HCV infection and hepatocellular carcinoma resulted in a broad differential diagnosis, including metastatic malignancies, recurrent hepatocellular carcinoma/dysplastic nodules, benign liver parenchymal lesions (e.g. nodular regenerative hyperplasia), and de novo malignancies (PTLD, Kaposi sarcoma, among others). The diagnosis of PTLD was not extremely challenging based on the morphology, especially if one could think of ordering an EBER (Epstein-Barr virus-encoded RNA) on the core specimen.
The role of chronic HCV infection is interesting in this case. As a well-established etiology for hepatocellular carcinoma, the virus is also reported as a risk factor for developing PTLD. HCV manifests with both hepatic and extrahepatic manifestations, including hematological, neurological, renal and dermatological disorders (6). HCV has been associated with expansion of B cell clones, the production of cryoglobulinemia and frank lymphomas (7, 8). A meta-analysis that included 48 studies concluded that the prevalence of HCV in patients with B-cell non-Hodgkin lymphoma was 15%, higher than that in the general population (approximately 1.5%) or in patients with other hematologic malignancies (3%) (8). In addition, some evidence suggested a decreased risk of lymphoma following treatment of HCV infection, and rare reports indicated regression of lymphoma with antiviral treatment.
The WHO classification of tumors of the hematopoietic and lymphoid tissues employs morphologic, immunophenotypic, genetic, and clinical features to define five main categories of PTLD: plasmacytic hyperplasia and infectious mononucleosis-like PTLD, florid follicular hyperplasia, polymorphic PTLD, monomorphic PTLD, and classic Hodgkin lymphoma-like PTLD. Once diagnosed, the outcome in transplant recipients appears to be worse than that in the general population (9).
Learning points
- PTLD is a lymphoplasmacytic disease that may occur after any type of organ transplant in the setting of immunosuppression. More than half of PTLD presents with extranodal masses.
- Multiple risk factors contribute to the development of PTLD, including the degree of immunosuppression and EBV status.
- HCV is also reported as a risk factor for developing PTLD. The infection manifests both hepatic and extrahepatic manifestations, and has been associated with expansion of B cell clones, the production of cryoglobulinemia and frank lymphomas.
- PTLD has been classified into five major groups by WHO book, and was reported carrying a worse outcome than that in the general population.
References
1. Nalesnik MA, Starzl TE. Epstein-Barr virus, infectious mononucleosis, and posttransplant lymphoproliferative disorders. Transplant Sci. 1994;4(1):61-79.
2. Aucejo F, Rofaiel G, Miller C. Who is at risk for post-transplant lymphoproliferative disorders (PTLD) after liver transplantation? J Hepatol. 2006;44(1):19-23.
3. Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222-30.
4. McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, et al. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. Am J Transplant. 2008;8(5):984-9.
5. Smets F, Sokal EM. Epstein-Barr virus-related lymphoproliferation in children after liver transplant: role of immunity, diagnosis, and management. Pediatr Transplant. 2002;6(4):280-7.
6. Shpilberg O, Wilson J, Whiteside TL, Herberman RB. Pre-transplant immunological profile and risk factor analysis of post-transplant lymphoproliferative disease development: the results of a nested matched case-control study. The University of Pittsburgh PTLD Study Group. Leuk Lymphoma. 1999;36(1-2):109-21.
7. Michaelis S, Kazakov DV, Schmid M, Dummer R, Burg G, Kempf W. Hepatitis C and G viruses in B-cell lymphomas of the skin. J Cutan Pathol. 2003;30(6):369-72.
8. Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125(6):1723-32.
9. Herrero JI. De novo malignancies following liver transplantation: impact and recommendations. Liver Transpl. 2009;15 Suppl 2:S90-4.
