Interesting Case May 2023
Case contributed by
Sadhna Dhingra M.D. Houston Methodist Hospital, Houston, Texas, USA
Parul Sobti M.D. Indraprastha Apollo Hospital, Delhi, India
Case History
A 65-year-old woman who presented for a gastric bypass revision and was found to have a 6.5 cm mass in the right lobe of liver. The intra-operative frozen section evaluation of liver biopsy demonstrated adenocarcinoma. A repeat liver biopsy was done 2 weeks later and showed a well to moderately differentiated adenocarcinoma. Immunohistochemical work-up showed tumor positive for CK 7, CK 19, and CDX-2. The tumor was negative for TTF-1, CK 20, glypican 3, Hepar-1, arginase 1, monoclonal CEA, PAX-8, GATA-3, mammaglobin, estrogen receptor, and progesterone receptor. Clinical and imaging work-up was negative for an extra-hepatic primary. Based on immunohistochemical results and imaging work-up, the tumor was favored to be an intrahepatic cholangiocarcinoma, and an extended right hepatectomy with cholecystectomy and portal lymphadenectomy was performed. Images of the resection specimen are as follows.
Pathology Findings
Gross examination showed a 6.5 cm well circumscribed white solid mass at the periphery of liver (Fig. 1). No cystic component was appreciated grossly. Microscopically, the main mass of the tumor was composed of small tubules and complex anastomosing tubules/glands in a sclerotic stroma (Fig. 2). Focal cribriform architecture was seen. The tubules were lined by atypical cuboidal to low columnar epithelium. Frequent mitoses were seen. Immunohistochemical work-up showed tumor positive for CK7, CK19 and negative for CK20. The subcapsular portion of tumor showed a Von Meyenburg Complex (VMC), characterized by ectatic and anastomosing tubules lined by bland cuboidal epithelium surrounded by a sclerotic stroma with mild chronic inflammation (Figs. 3 and 4). No nuclear atypia or mitosis was seen in the VMC. The size of VMC was estimated to be at least 3 cm. The VMC transitioned into atypical adenomatous proliferation characterized by complex interconnecting tubules lined by mildly atypical low cuboidal epithelium with occasional mitoses (Figs. 5 and 6). The Ki-67 immunostaining was helpful in delineating the zone of atypical adenomatous proliferation from VMC and cholangiocarcinoma (Figs. 7, 8 and 9). P53 immunostain showed a wild type expression pattern in VMC, and focal moderate to strong nuclear staining in the cholangiocarcinoma. No vascular invasion was seen. The resected lymph nodes were negative for metastatic adenocarcinoma. The tumor was staged as stage 1 (pT1b) as per AJCC/CAP 8th edition staging protocol.
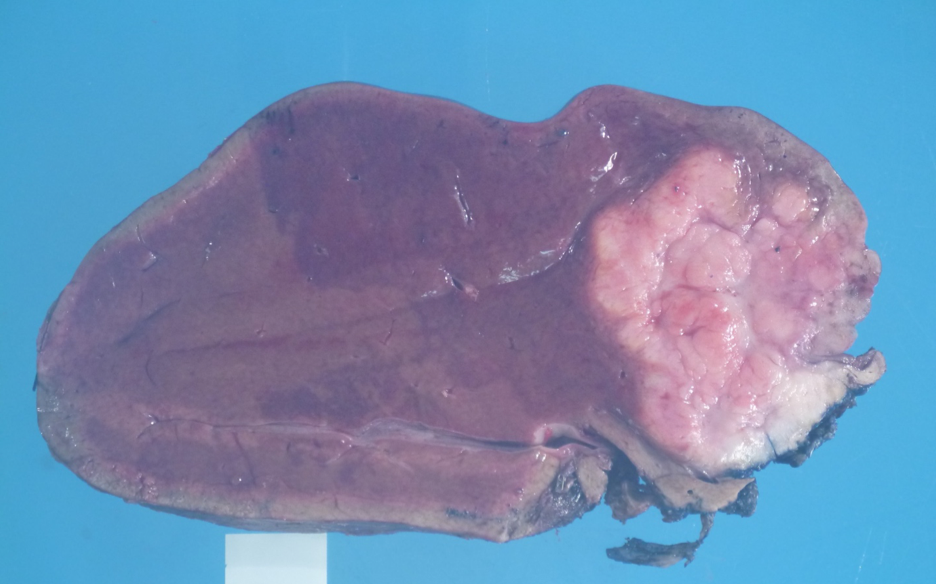
Fig 1. Gross image of liver mass
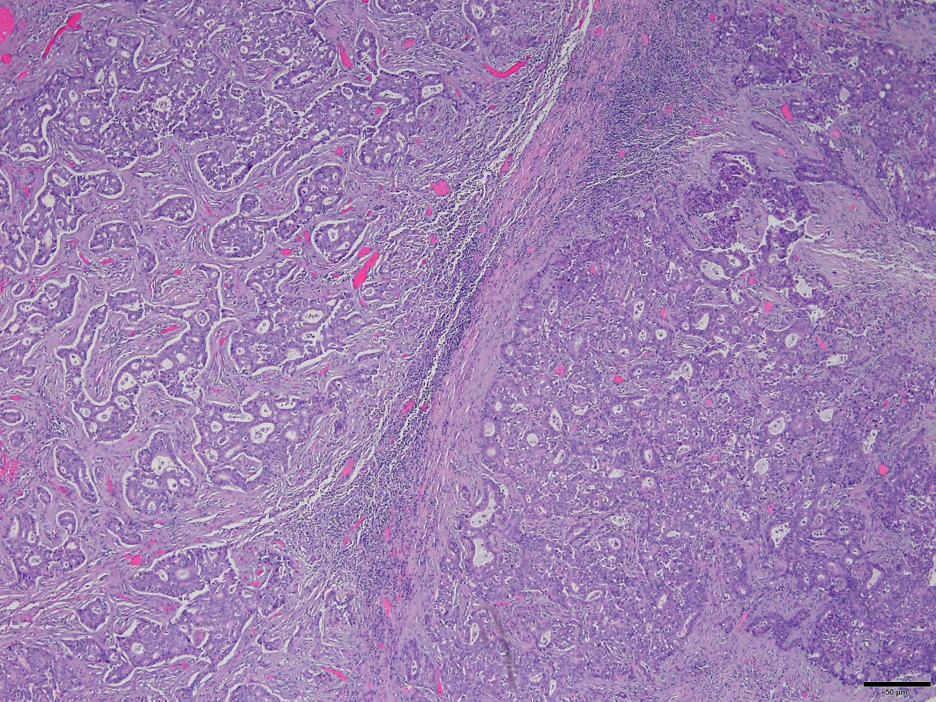
Fig. 2. Cholangiocarcinoma. Hematoxylin and eosin stain. 20x
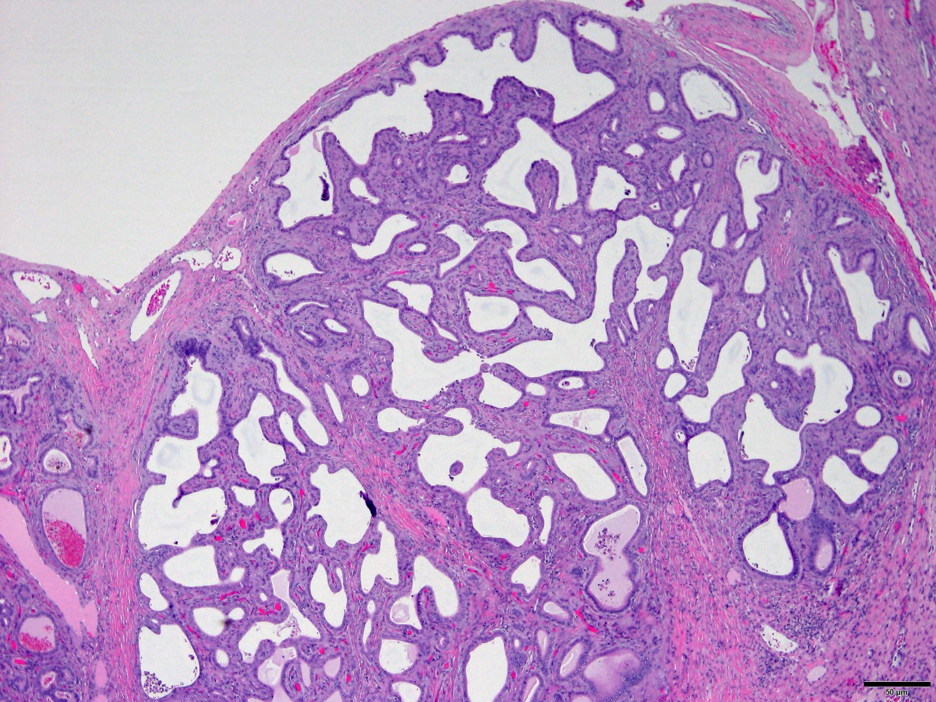
Fig 3. Subcapsular Von Meyenburg Complex. Hematoxylin and Eosin stain. 20x
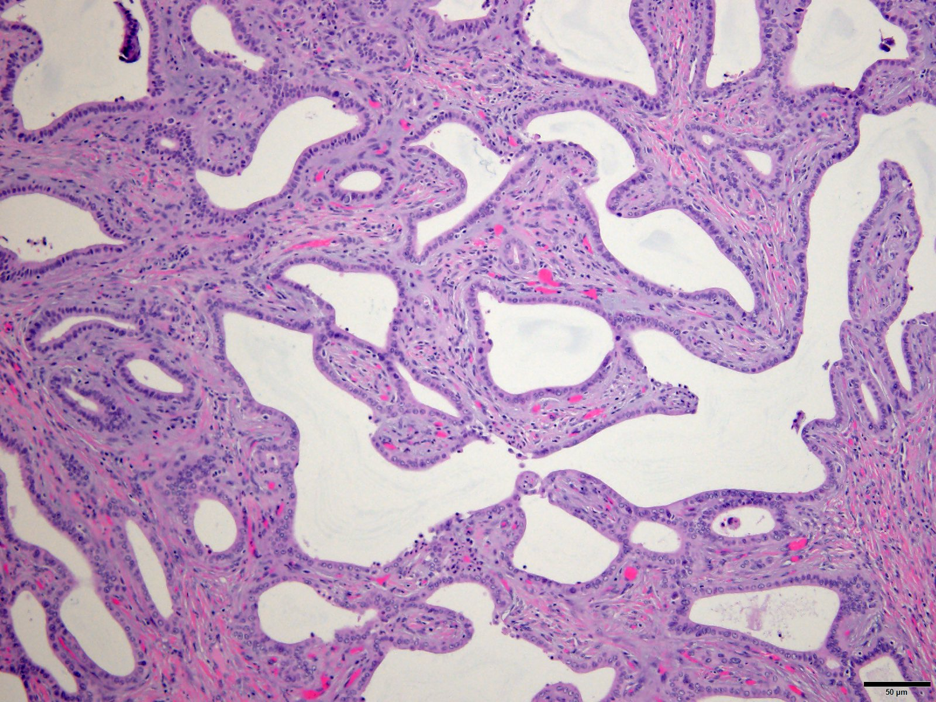
Fig. 4. Von Meyenburg Complex composed of dilated ectatic angulated glands in a fibrotic and inflamed stroma. Hematoxylin and Eosin stain. 40x.
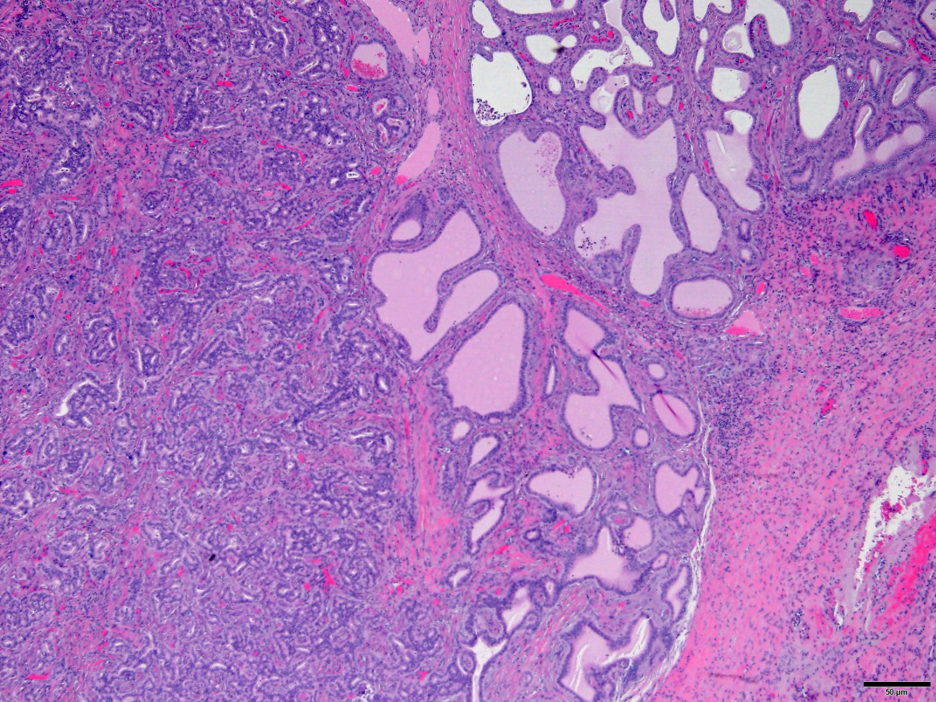
Fig. 5. Interface of Von Meyenburg Complex and atypical adenomatous proliferation. Hematoxylin and Eosin stain. 20x
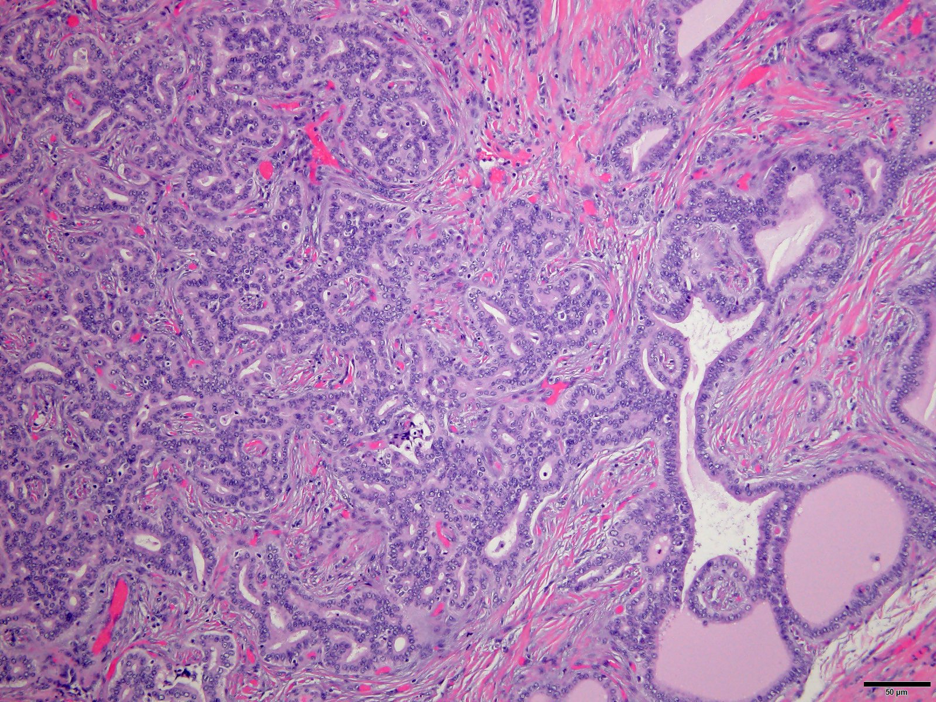
Fig. 6. Interface of Von Meyenburg Complex and atypical adenomatous proliferation. Hematoxylin and Eosin stain. 40x
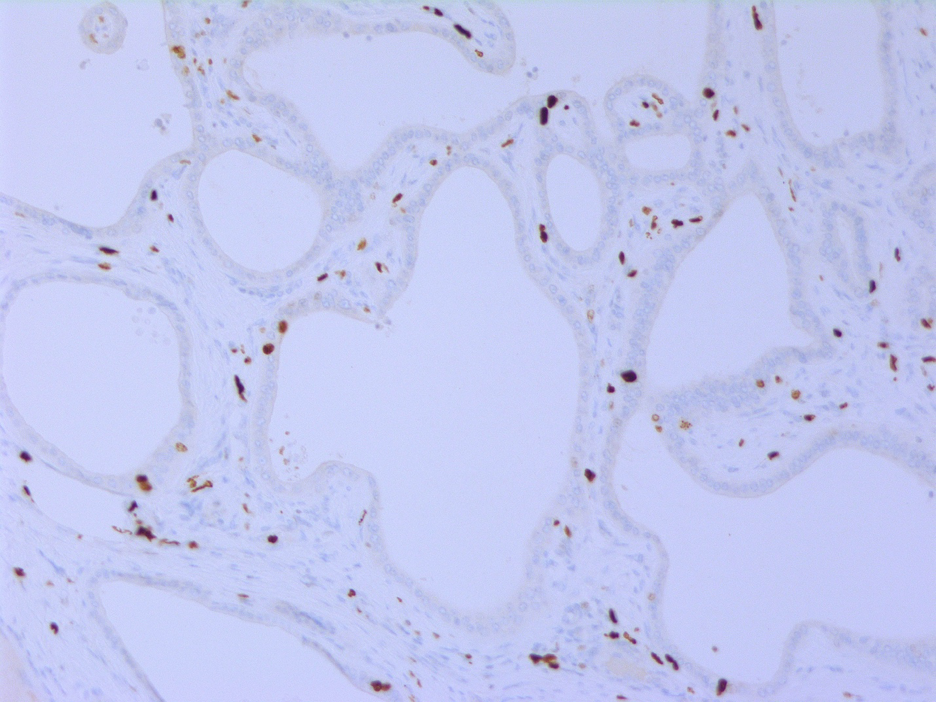
Fig 7. Ki-67 immunostain in the Von Meyenburg Complex showing occasional scattered positive cells (proliferation index 1%).
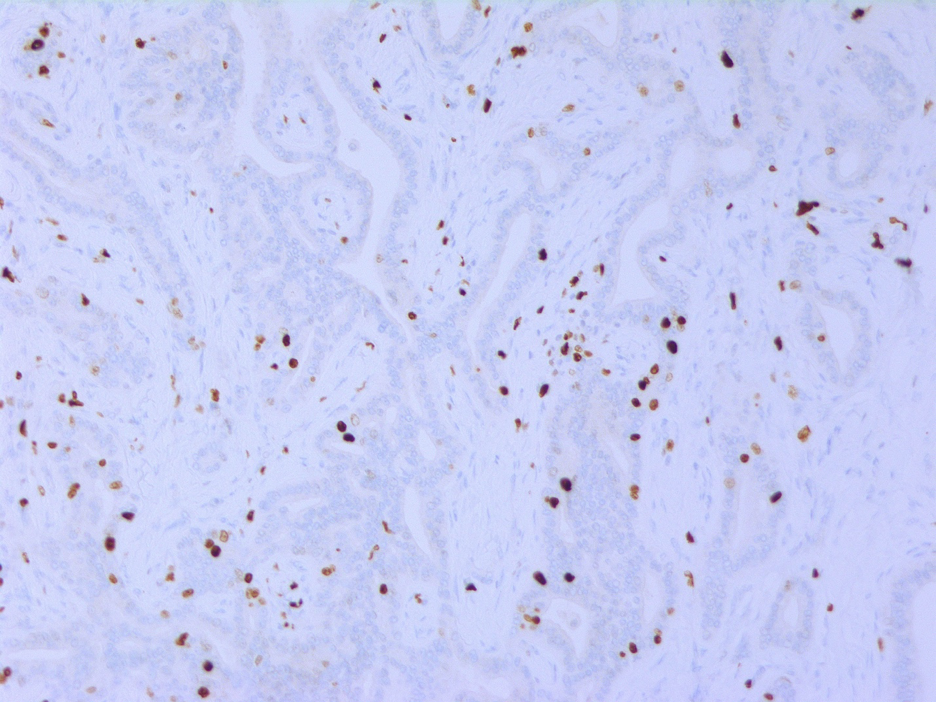
Fig 8. Ki-67 immunostain in the focus of atypical adenomatous proliferation showing increased proliferation rate (PI 5%)
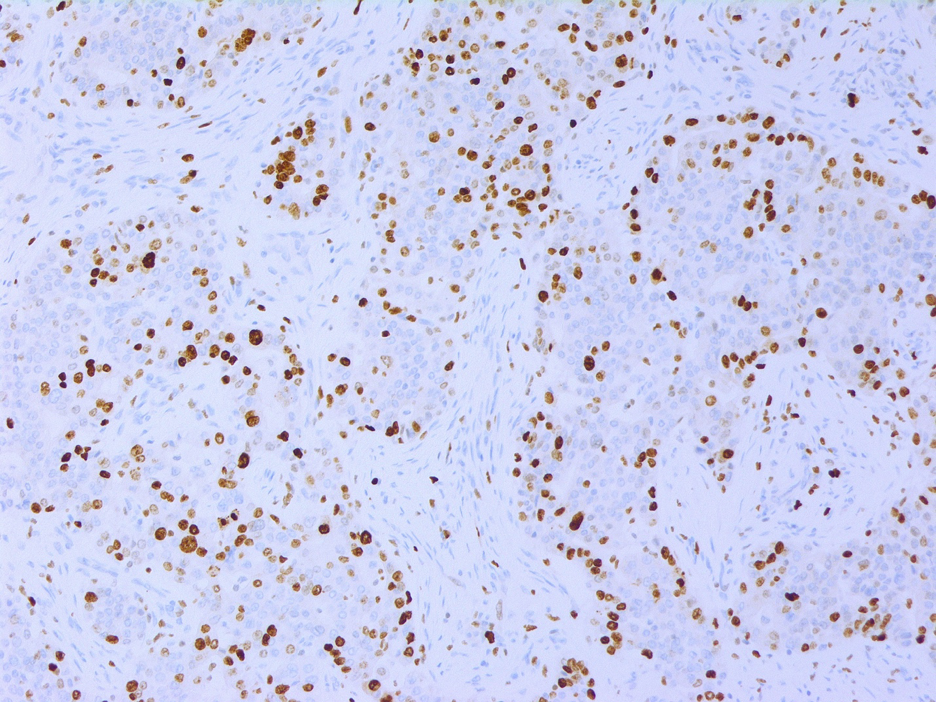
Fig 9. Ki-67 immunostain in the cholangiocarcinoma showing high proliferation index (40%)
Diagnosis
Intrahepatic Cholangiocarcinoma arising in a Von Meyenburg complex.
Follow up: Patient died of metastatic disease 2 years after diagnosis.
Discussion
Intrahepatic cholangiocarcinoma (ICC) is a rare malignancy and makes up 8% to 10% of cholangiocarcinoma, and 10% to 20% of all primary tumors (1). ICC is of large duct type and small duct type. Large duct intrahepatic cholangiocarcinoma is located closer to hilum, spreads along the large portal tracts with periductal infiltrating pattern and resembles extrahepatic cholangiocarcinoma. Small duct intrahepatic cholangiocarcinoma is peripheral in location and presents as a mass. Intrahepatic cholangiocarcinoma with ductal plate formation-like pattern is a subtype of small duct cholangiocarcinoma.
Ductal plate malformation results from absence of remodeling of the embryonic ductal plate during bile duct morphogenesis. This results in several clinicopathologic phenotypes such as congenital hepatic fibrosis, Caroli’s disease, Von Meyenburg complex (VMC) and autosomal polycystic kidney and liver diseases (dominant and recessive variants). Von Meyenburg complex is a small, usually less than 1.5 cm, benign bile duct lesion which is often subcapsular in location. It was first described by Moschcowitz in 1906 as aberrant bile ducts due to persistent embryonic state (2). This was then named Von Meyenburg complex after a Swiss pathologist, Hans Von Meyenburg, who reported it in 1918 (3). It is also popularly known as biliary hamartoma. As demonstrated in our case, VMC is composed of dilated and anastomosing biliary tubules lined bland cuboidal epithelium with a very low proliferative index, surrounded by a sclerotic stroma. Intraluminal bile or proteinaceous secretion is commonly seen in these tubules.
The risk of malignant transformation of VMC is small. However, the true incidence is not known because the ICC might completely replace the precursor lesion, if present, or the precursor lesion may be focal and not sampled. Majority of malignancies arising in VMC are cholangiocarcinoma. Evidence of gradual neoplastic progression of VMC from benign to atypical adenomatous proliferation or biliary dysplasia to cholangiocarcinoma has been demonstrated with the use of Ki-67 and p53 immunostains (4-8). Jain et al (9) demonstrated loss of heterozygosity (LOH) at key genetic loci in VMC, similar to ICC, in 2 cases, and suggested that VMC likely harbor mutations in key tumor suppressor genes that progress with time to intermediate stage and then cholangiocarcinoma. Further studies are needed to substantiate this finding. Long standing cholestasis, dilatation, inflammation and hepatotoxins may have a role to play in the carcinogenesis in VMCs. Rare cases of Hepatocellular carcinoma arising in association with VMC have been reported (10,11). Song et al have suggested an association with size or number of VMC and development of malignancy (11).
Radiologically, the major differential diagnosis of VMC are hepatic metastases and micro-abscesses (12). Histologically, the differential includes a bile duct adenoma. Bile duct adenoma (BDA) is morphologically distinguished from VMC by presence of small round tubules with inconspicuous lumens. No intraluminal bile is seen in the BDA.
Large VMCs as large as 20 cm have been reported in literature and are referred to as giant cystic VMC/BDH (13,14). These may represent biliary adenofibroma, a rare solid and cystic hepatic neoplasm which shows morphologic similarity to VMC but has been determined to be a neoplasm (15-17). The cuboidal epithelium of VMC and biliary adenofibroma share the same expression profile: foregut antigen D10 (+) and IF6 (-). Biliary adenofibroma is recognized as a distinct primary hepatic neoplasm in the 2010 WHO classification of hepatic tumors.
Learning Points
- Von Meyenburg complex is a benign lesion with a malignant potential.
- The most common malignancy is intrahepatic cholangiocarcinoma, however, rare cases of hepatocellular carcinoma have also been reported.
- Studies have demonstrated evidence of neoplastic progression from VMC to atypical adenomatous proliferation and cholangiocarcinoma.
- Giant cystic Von Meyenburg Complex may represent biliary adenofibroma, rare solid and cystic hepatic neoplasm.
References
- Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017 Apr;6(2):101-104.
- Moschcowitz E. Non-parasitic cysts (congenital) of the liver with a study of aberrant bile ducts. Am J Med Sci 1906; 131: 674-699.
- von Meyenburg H. About the liver cyst. Beitr Pathol Anat. 1918;64:477–535.
- Bhalla A, Mann SA, Chen S, Cummings OW, Lin J. Histopathological evidence of neoplastic progression of von Meyenburg complex to intrahepatic cholangiocarcinoma. Hum Pathol. 2017 Sep;67:217-224.
- Wang Q, Xu Y, Wang SM, Hu AY, Pan YC, Zhang SH. Histopathological evidence of intrahepatic cholangiocarcinoma occurring in ductal plate malformation: A clinicopathologic study of 5 cases. Ann Diagn Pathol. 2021 Dec;55:151828.
- Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, Uehara T, Yamamoto M, Ariizumi S, Park YN, Choi JH, Yu E. Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: a new subtype. Am J Surg Pathol. 2012 Nov;36(11):1629-35.
- Jain D, Sarode VR, Abdul-Karim FW, Homer R, Robert ME. Evidence for the neoplastic transformation of Von-Meyenburg complexes. Am J Surg Pathol. 2000 Aug;24(8):1131-9.
- Tsokos CG, Krings G, Yilmaz F, Ferrell LD, Gill RM. Proliferative index facilitates distinction between benign biliary lesions and intrahepatic cholangiocarcinoma. Hum Pathol. 2016 Nov;57:61-67.
- Jain D, Ahrens W, Finkelstein S. Molecular evidence for the neoplastic potential of hepatic Von-Meyenburg complexes. Appl Immunohistochem Mol Morphol. 2010 Mar;18(2):166-71.
- Heinke T, Pellacani LB, Costa Hde O, Fuziy RA, Franco M. Hepatocellular carcinoma in association with bile duct hamartomas: report on 2 cases and review of the literature. Ann Diagn Pathol. 2008 Jun;12(3):208-11.
- Song JS, Lee YJ, Kim KW, Huh J, Jang SJ, Yu E. Cholangiocarcinoma arising in von Meyenburg complexes: report of four cases. Pathol Int. 2008 Aug;58(8):503-12. doi: 10.1111/j.1440-1827.2008.02264.x. PMID: 18705771.
- Zheng RQ, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol. 2005 Oct 28;11(40):6354-9.
- Singh Y, Cawich SO, Ramjit C, Naraynsingh V. Rare liver tumor: symptomatic giant von Meyenburg complex. J Surg Case Rep. 2017 Jan 9;2016(11):rjw195.
- Redston MS, Wanless IR. The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies [corrected]. Mod Pathol. 1996 Mar;9(3):233-7. Erratum in: Mod Pathol 1996 Jul;9(7):803. PMID: 8685220.
- Torbenson MS. Hamartomas and malformations of the liver. Semin Diagn Pathol. 2019 Jan;36(1):39-47.
- Varnholt H, Vauthey JN, Dal Cin P, Marsh Rde W, Bhathal PS, Hughes NR, Lauwers GY. Biliary adenofibroma: a rare neoplasm of bile duct origin with an indolent behavior. Am J Surg Pathol. 2003 May;27(5):693-8.
- Arnason T, Borger DR, Corless C, Hagen C, Iafrate AJ, Makhlouf H, Misdraji J, Sapp H, Tsui WM, Wanless IR, Zuluaga Toro T, Lauwers GY. Biliary Adenofibroma of Liver: Morphology, Tumor Genetics, and Outcomes in 6 Cases. Am J Surg Pathol. 2017 Apr;41(4):499-505.
