Interesting Case October 2022
Case contributed by:
Jane Lee, MD, PGY-4 Pathology Resident, and Kathleen Byrnes, MD, Assistant Professor
Department of Pathology & Immunology at Washington University School of Medicine
Have an interesting case to share?
Contact HPHS newsletter committee chair Sadhna.Dhingra@ProPath.com
Case history
A 43 year old woman with a past medical history of total pancreatectomy and auto islet cell transplant for presumed chronic pancreatitis. She presented with abdominal pain, bloating, nausea, and diarrhea. Her lab workup was significant for elevated alkaline phosphatase 203 Units/L (reference 40-130), AST 75 Units/L (reference 10-45), ALT 50 Units/L (reference 7-45), total bilirubin 0.3 mg/dL (reference 0.1-1.2), and HbA1c 6.6% (reference 4.0-5.6). An MRI with MRCP revealed severe diffuse hepatic steatosis with no focal lesions. She underwent a liver biopsy to assess for liver fibrosis and assessment of steatosis.
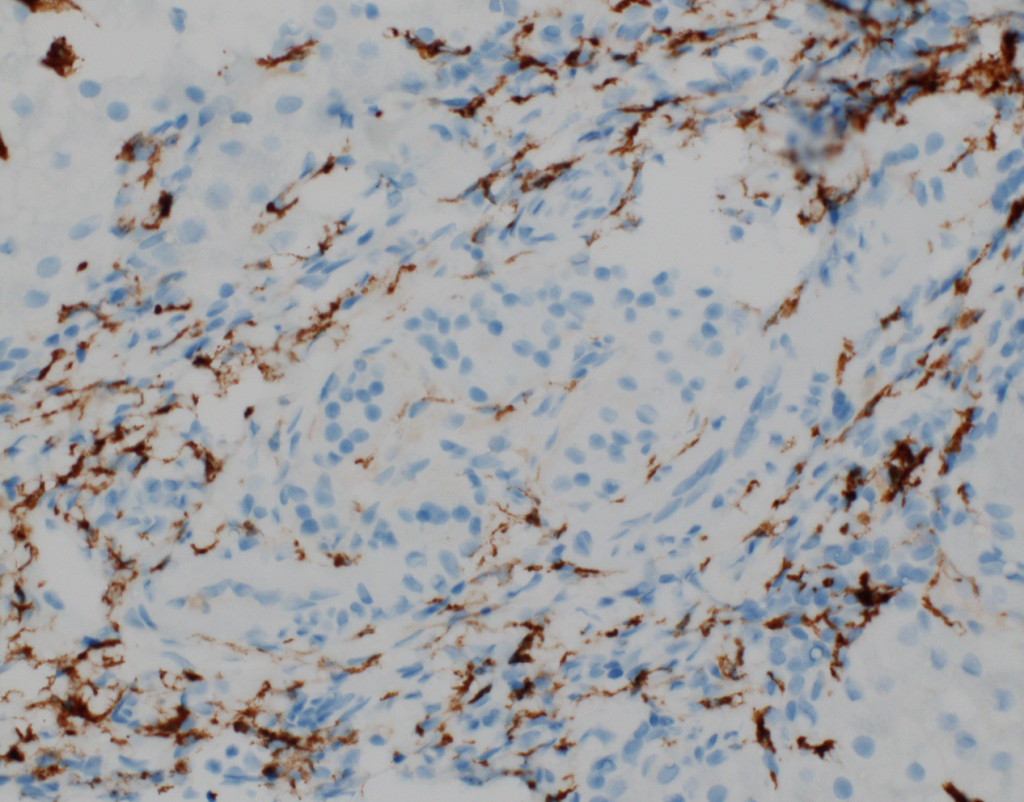
Pathologic findings
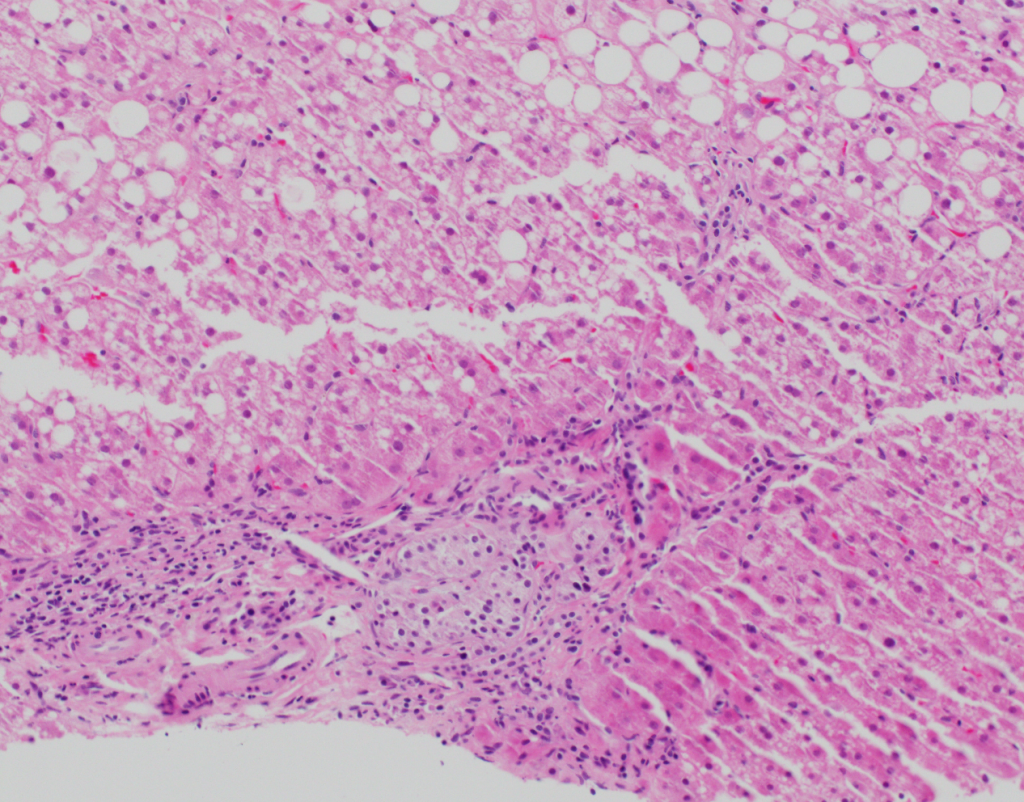
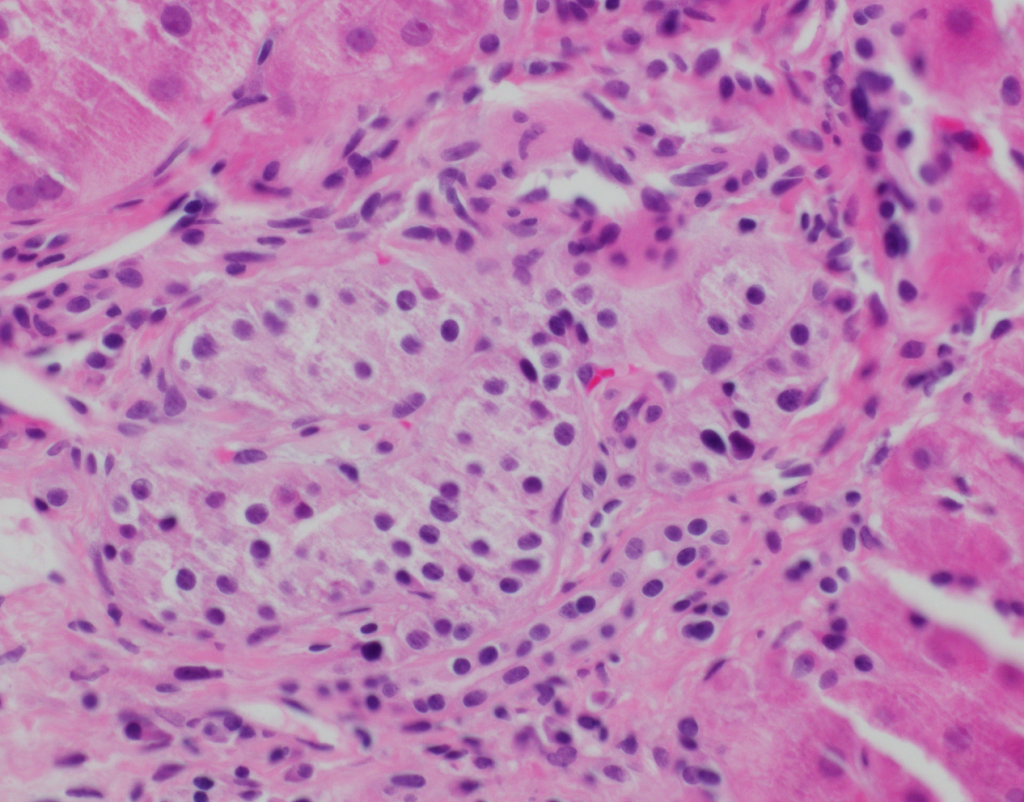
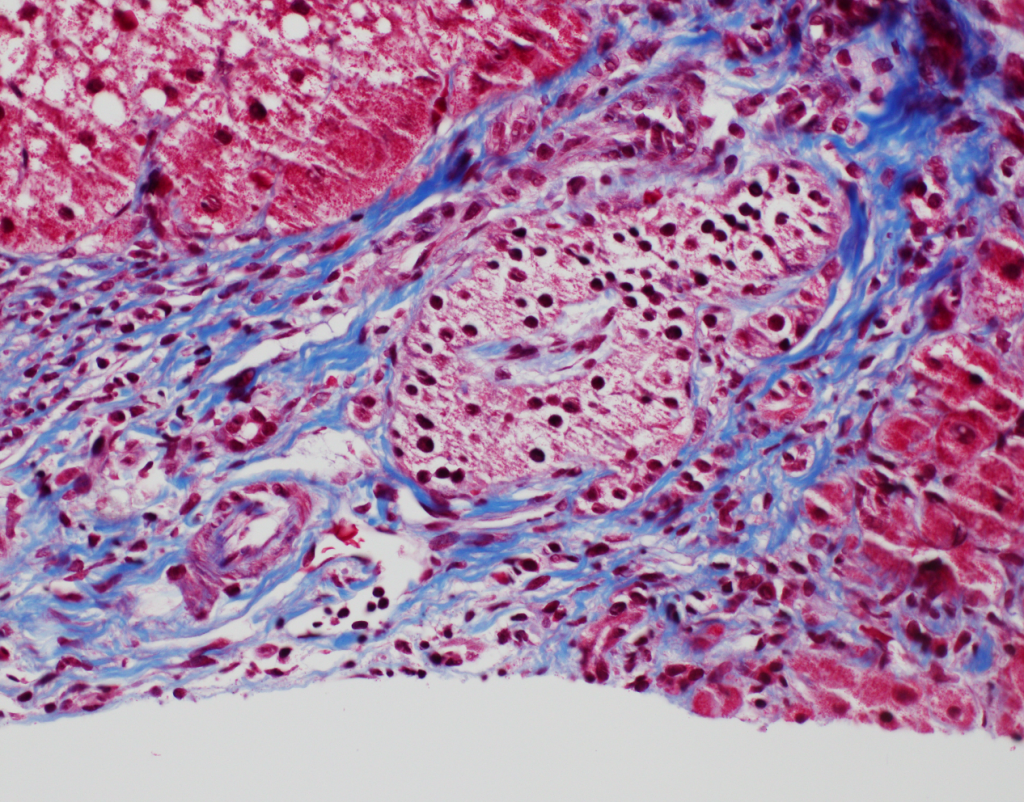
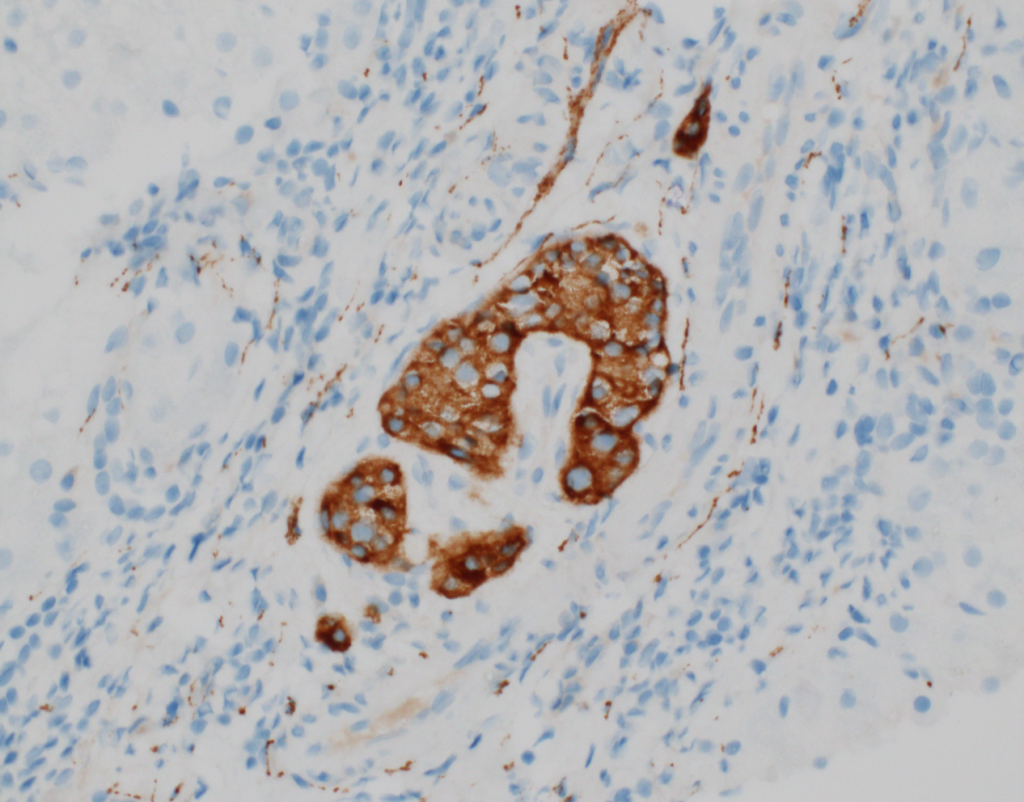
At low power, the liver has steatosis and the majority of portal tracts have intact bile ducts with no significant findings. There is minimal lobular inflammation and no ballooned hepatocytes are noted. Within a single portal tract, there is a nest of neuroendocrine cells noted within a vessel (Figures 1a and 1b). There are no mitotic figures noted within this nest of cells. Trichrome stain demonstrates no significant fibrosis, but confirms the presence of the neuroendocrine cells within a vascular space (Figure 2). Immunostains confirm neuroendocrine differentiation (Figures 3a and 3b).
Diagnosis
Islet cell transplantation
Discussion
Pancreatic islet cell transplant was first performed in diabetic mice, with islet cells implanted in various sites. Early studies demonstrated reversal of the diabetic state in cases of islet cell injection into the portal vein. Engrafted islet cells were found predominantly in the interlobular portal venules, and appeared structurally normal. Occasional islet cells could also be found outside portal areas directly adjacent to hepatocytes. Early in the post-transplant interval, hepatocytes demonstrated prominent cytoplasmic vacuolization, and were found to contain glycogen and lipid. Hepatocytes closest to the transplanted islet cells were most severely affected.
Intrahepatic islet cell transplant is an alternative to whole-pancreas transplantation in patients with type 1 diabetes or post-surgical diabetes. Advantages of engrafting islet cells intrahepatically include establishment of insulin delivery directly into the portal circulation, akin to insulin secretion in normal physiology. However, historically many islet cell graft recipients eventually develop insulin resistance years post-transplant.
Islet infusion triggers an inflammatory response, which activates the coagulation cascade and results in thrombotic complications. Steatosis is hypothesized to increase the inflammatory response and adversely affect islet cell engraftment and survival. Improvements in the efficiency of islet cell engraftment are leading to similar rates of insulin independence in follow-up studies.
Other differentials include metastatic well-differentiated neuroendocrine tumor. The liver is a common site of neuroendocrine tumor metastasis, with more than half of liver metastases from the small bowel. Well-differentiated neuroendocrine tumors are composed of uniform cells with round nuclei, finely granular chromatin, and eosinophilic cytoplasm. These can histologically show similarities to the islet cells if careful attention is not paid to the clinical history. The tumor can display variable architectural patterns, including trabecular, nested, and pseudoglandular. Classically neuroendocrine cells are positive for synaptophysin and chromogranin A. In this context, clinical history is extremely important. Recognizing the patient has a history of islet cell transplant can prevent misidentifying the neuroendocrine cells as a well-differentiated neoplasm.
Learning points:
- Patients with islet cell transplantation can present years after transplantation with residual islet cells.
- The morphologic overlap between islet cells transplanted in the liver and metastatic well-differentiated neuroendocrine tumors presents a pitfall if the clinical history is limited.
- Islet cells are usually periportal and can be located within vascular spaces.
- In the early post-transplant period, islet cells may elicit an inflammatory response in the adjacent hepatocytes.
References
- Griffith RC, Scharp DW, Hartman BK, Ballinger WF, Lacy PE. A Morphologic Study of Intrahepatic Portal-vein Islet Isografts. Diabetes. 1977; 26(3):201-214.
- Andersson A, Korsgren O, Jansson L. Revascularization of Islets: Intraportally Transplanted Pancreatic Islets Revascularized From Hepatic Arterial System. Diabetes. 1989; 38:192-195.
- Meier JJ, Hong-McAtee I, Galasso R, Veldhuis JD, Moran A, Hering BJ, Butler PC. Intrahepatic Transplanted Islets in Humans Secrete Insulin in a Coordinate Pulsatile Manner Directly Into the Liver. Diabetes. 2006; 55:2324-2332.
- Desai CS, Khan KM, Ma X, Li H, Wang J, Fan L, Chen G, Smith JP, Cui W. Effect of liver histopathology on islet cell engraftment in the model mimicking autologous islet cell transplantation. Islets. 2017; 9(6):140-140.
- Rickels MR, Robertson RP. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocrine Reviews. 2019; 40(2):631-668.
- Park JH, Kim JH. Pathologic differential diagnosis of metastatic carcinoma in the liver. Clinical and Molecular Hepatology. 2019; 25(1):12-20.
- Bellizzi AM. Pathologic Considerations in Gastroenteropancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am. 2020; 29(2):185-208.
