Interesting Case April 2022
Case contributed by:
Deyali Chatterjee, MD
Assistant Professor, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Have an interesting case to share?
Contact HPHS newsletter committee chair Sadhna.Dhingra@ProPath.com
Case history
A 63 year old woman with history of treated hepatitis C, completed 6 years ago, underwent a second open partial hepatectomy (seg VIII/V) for recurrent hepatocellular carcinoma (1.5 cm), after completing three cycles of neoadjuvant azetolizumab and bevacizumab. The liver enzymes, serum protein levels, and bilirubin level were normal; although the platelet count was low at 120 K/ microliter. Intraoperatively, the liver was noted to be firm, but without gross nodular appearance.
Following are the images from the background liver, greater than 2 cm from the tumor.
Fig 1A: HE stain
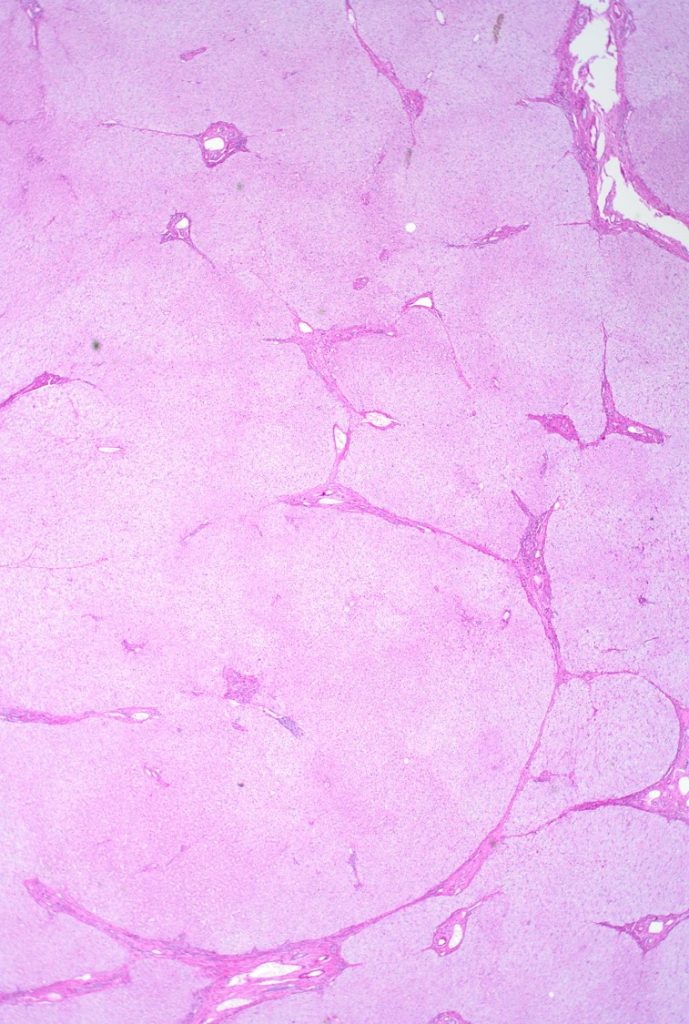
Fig 1B: trichrome stain
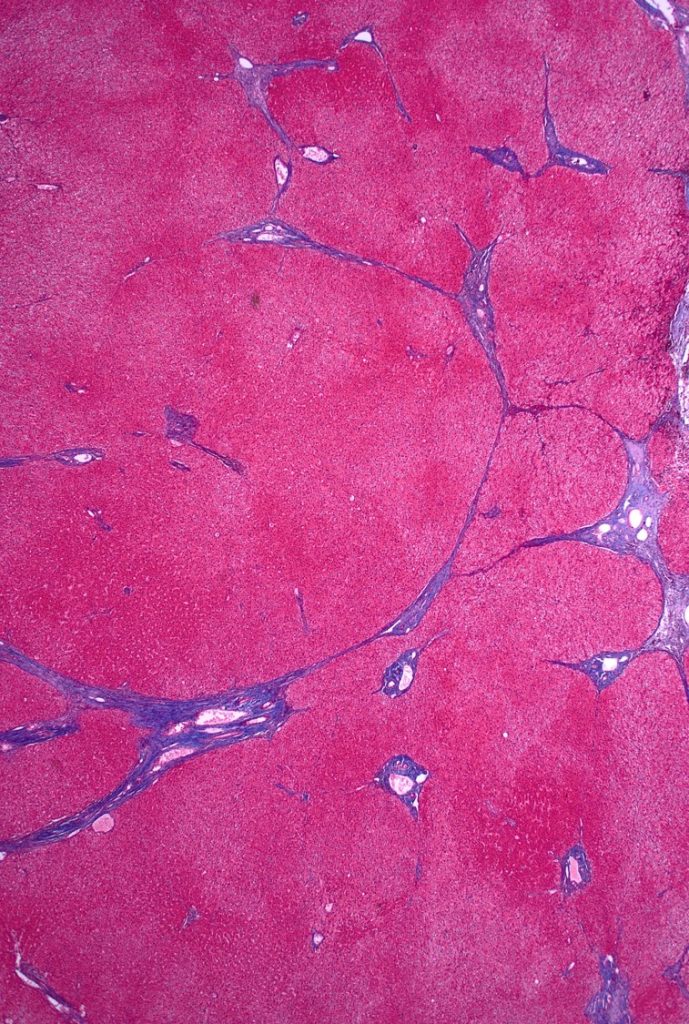
Fig 2A: trichrome stain
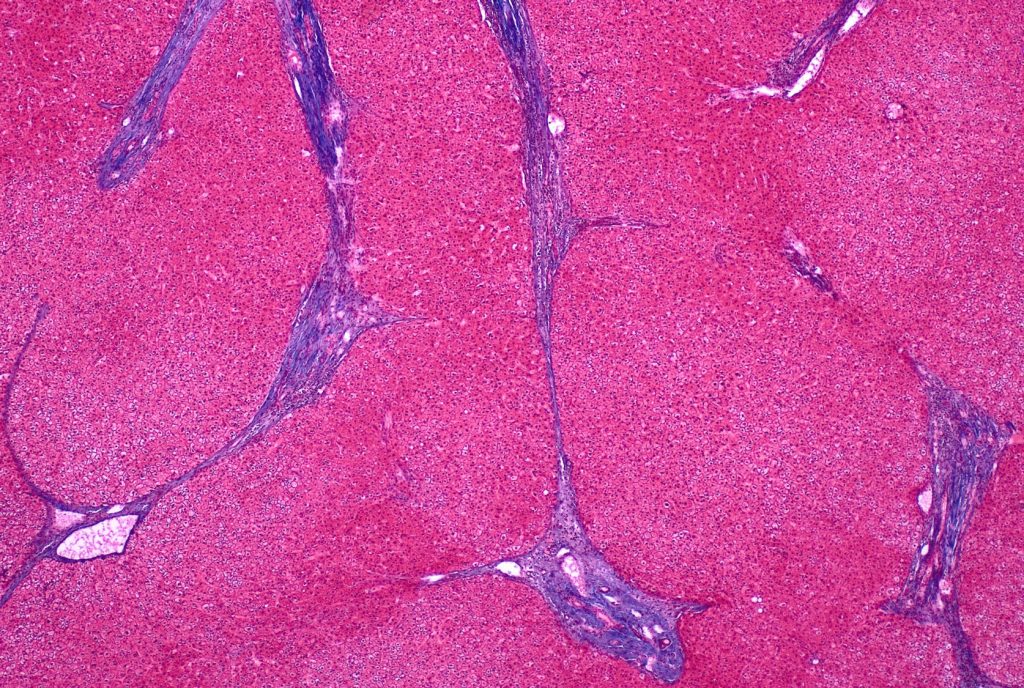
Fig 3: HE stain
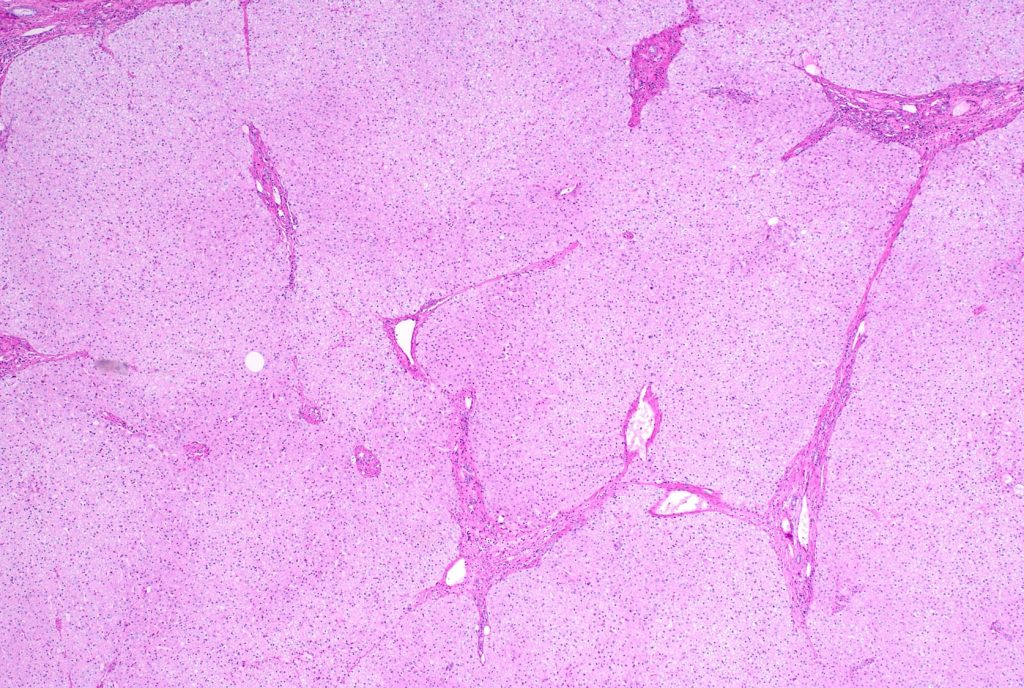
Figure 4
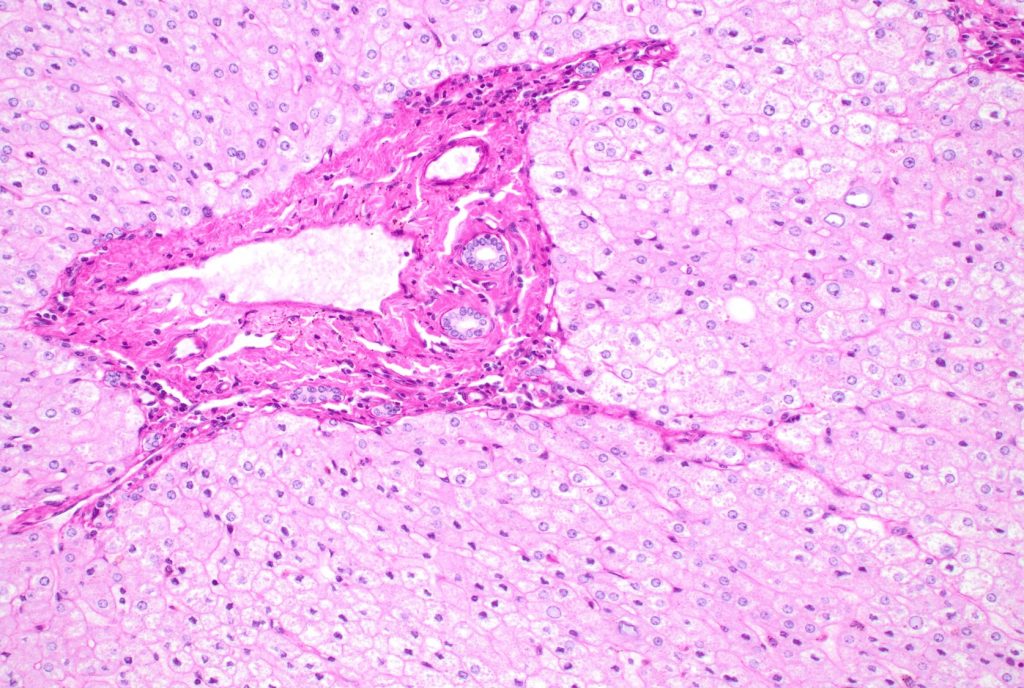
Fig 5
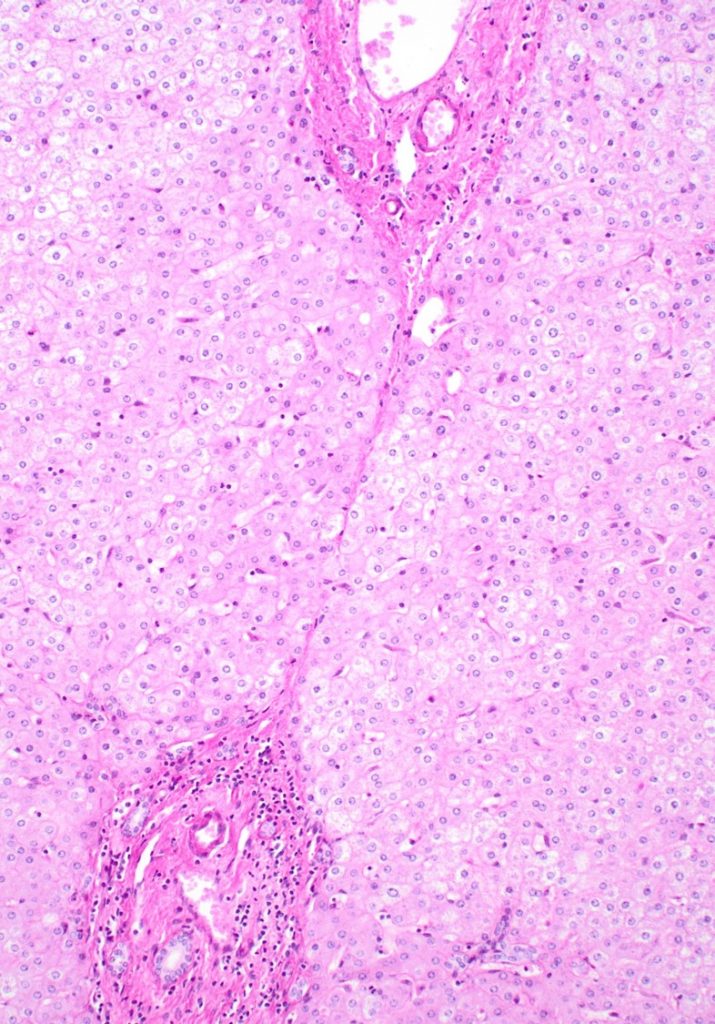
Fig 6
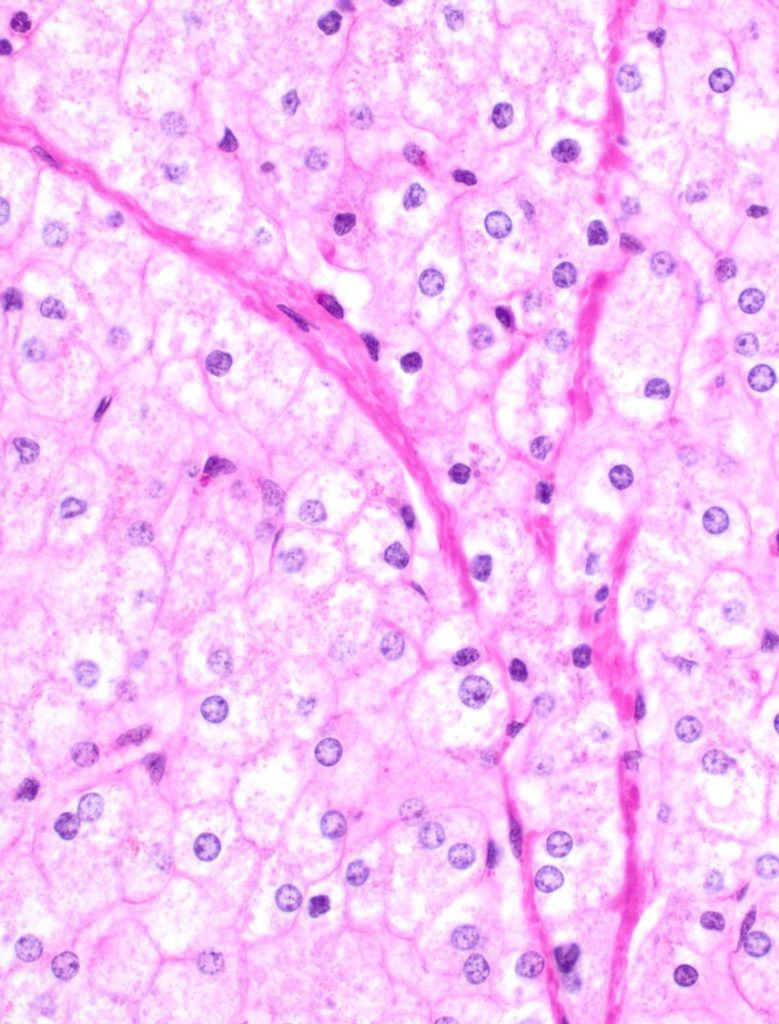
Fig 6: trichrome stain
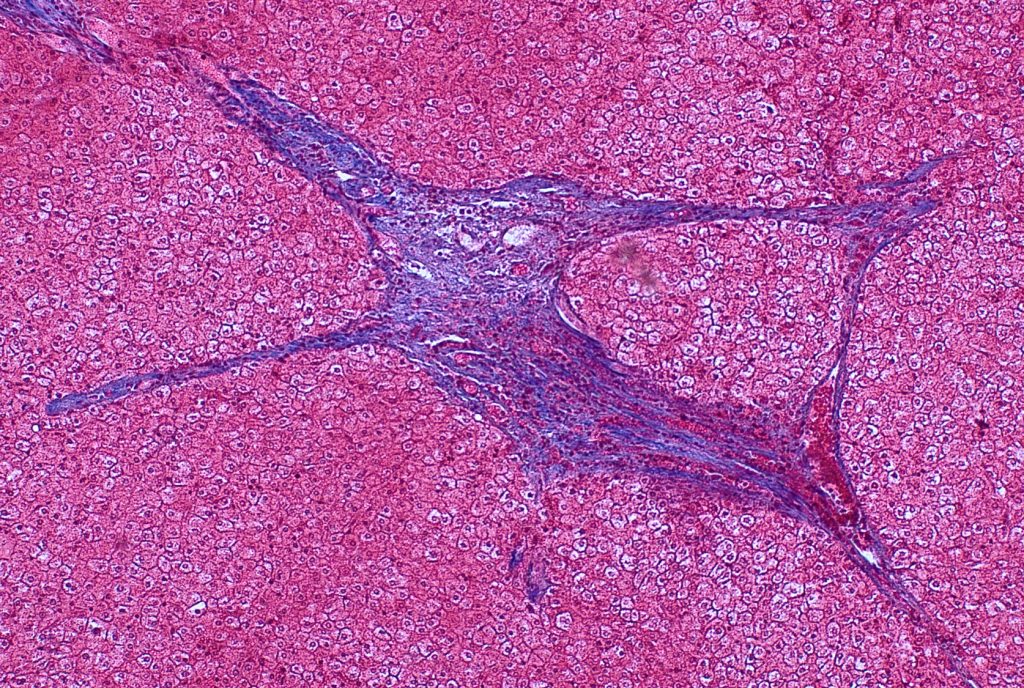
Fig 7
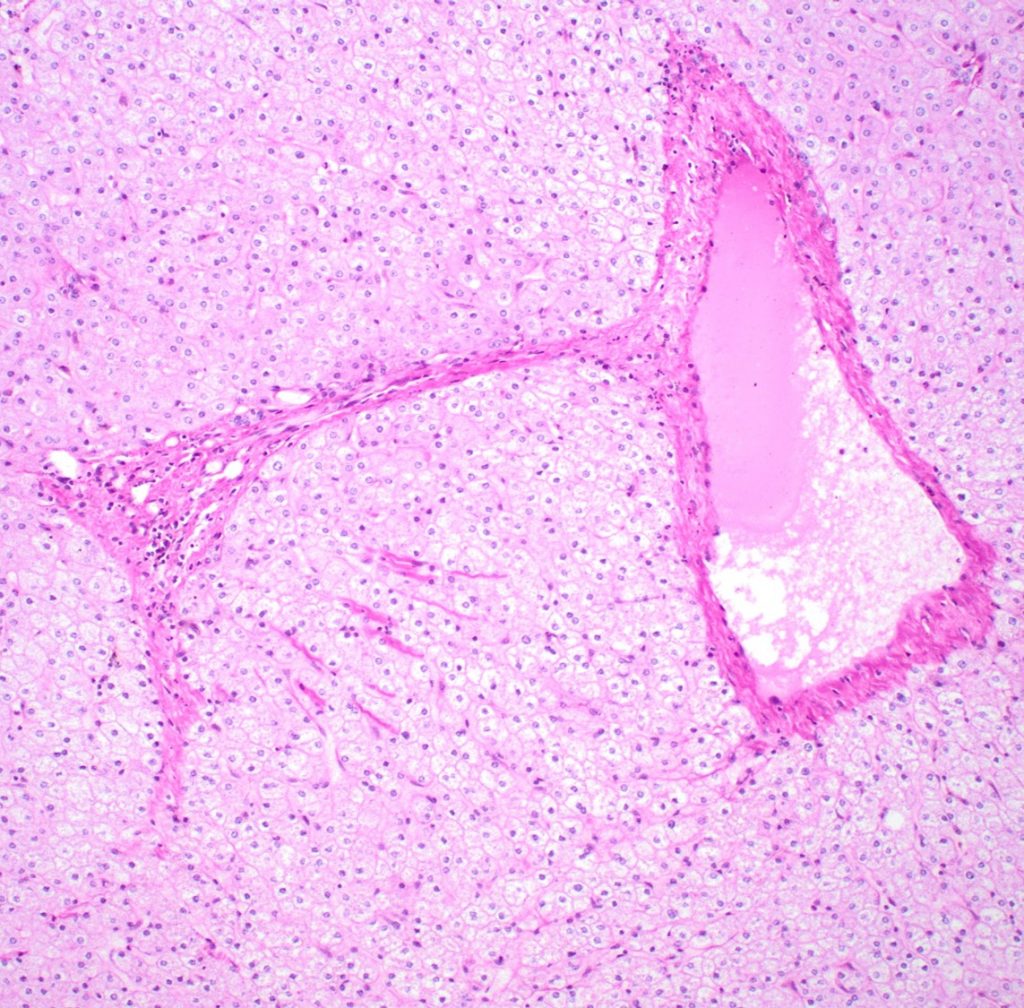
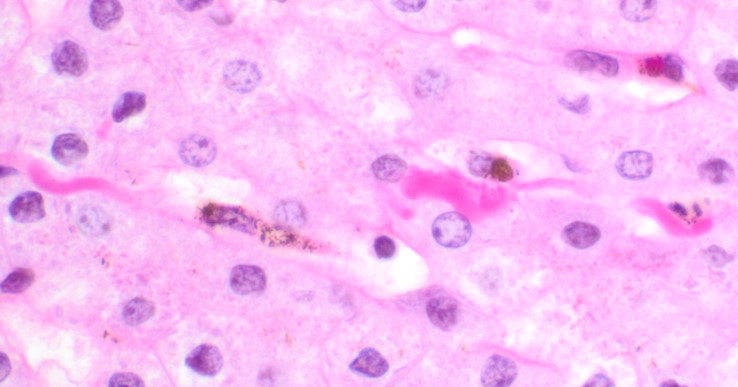
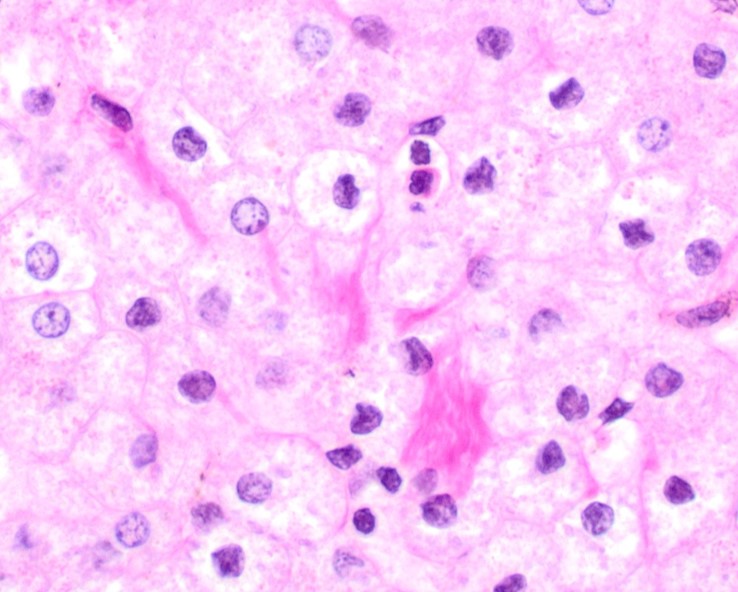
Fig 8
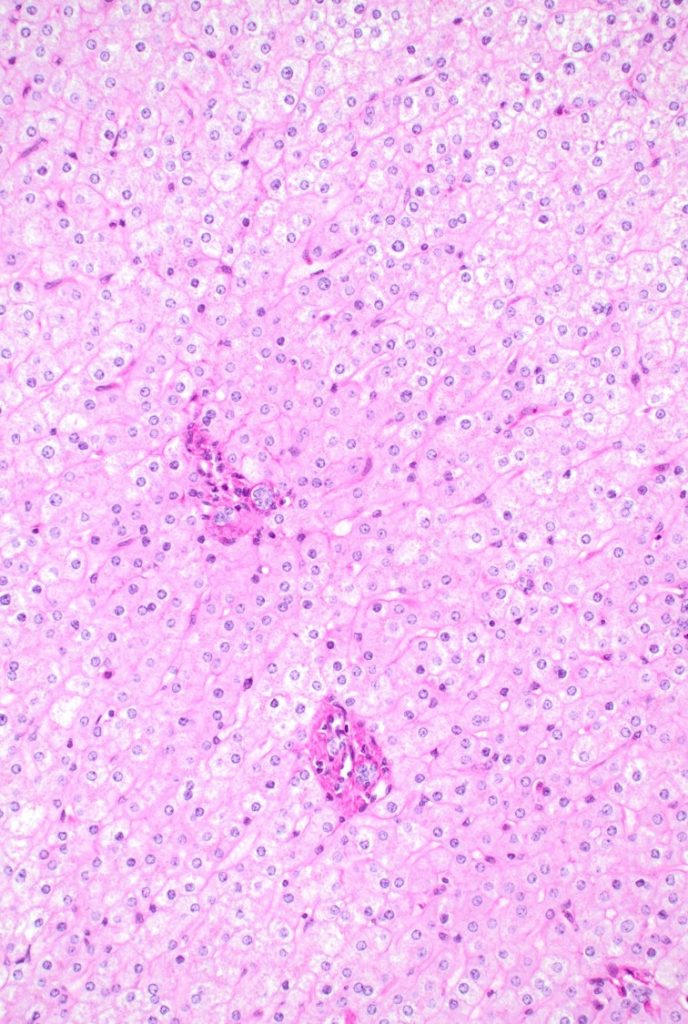
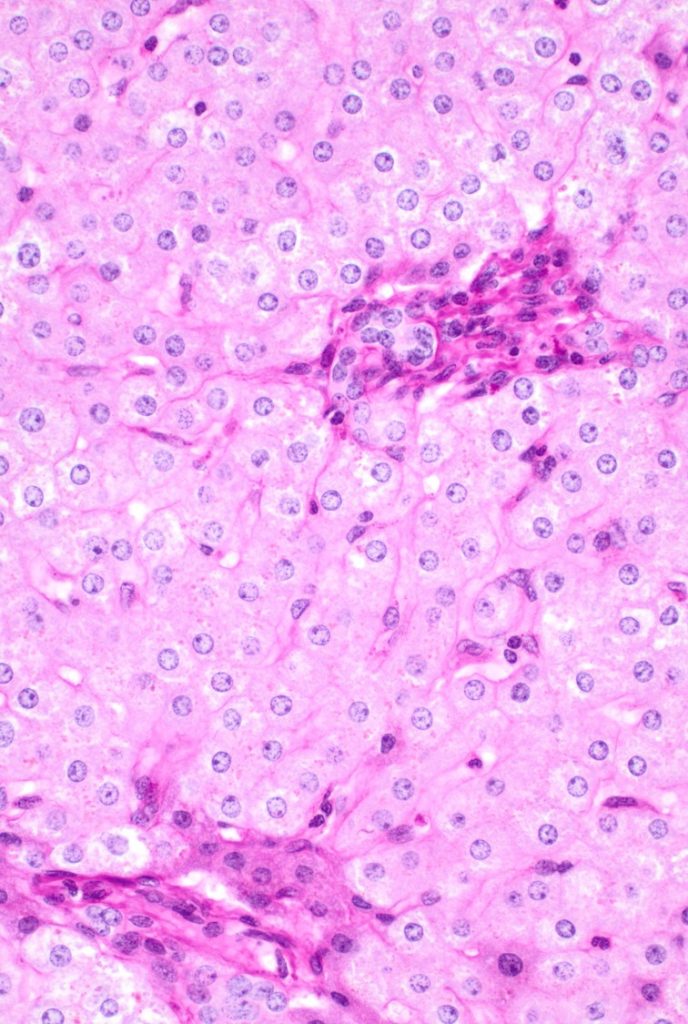
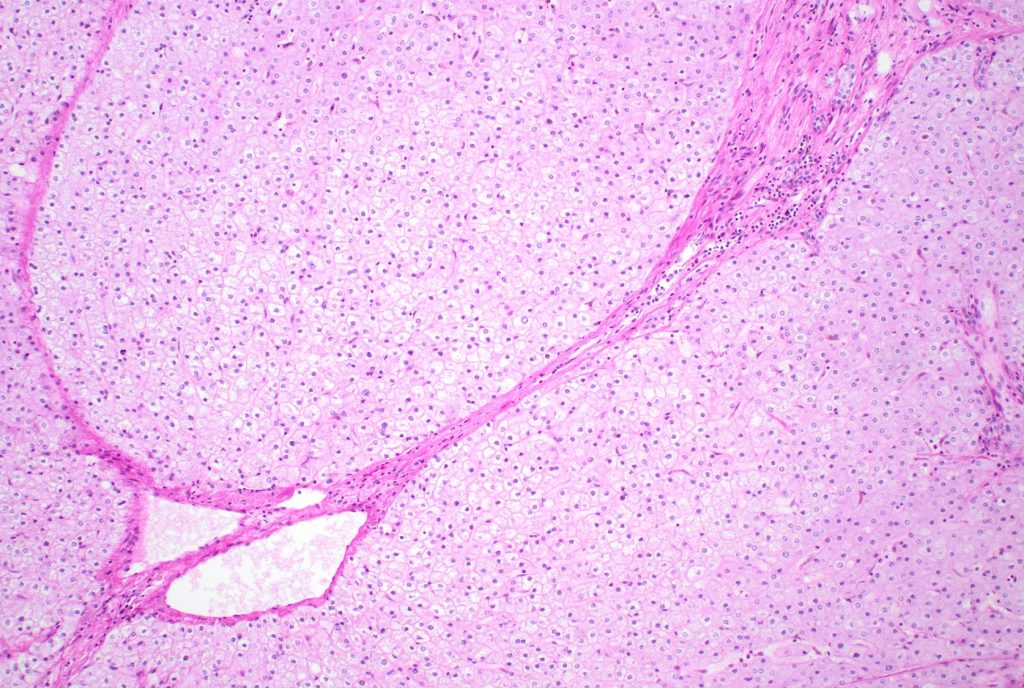
Fig 9
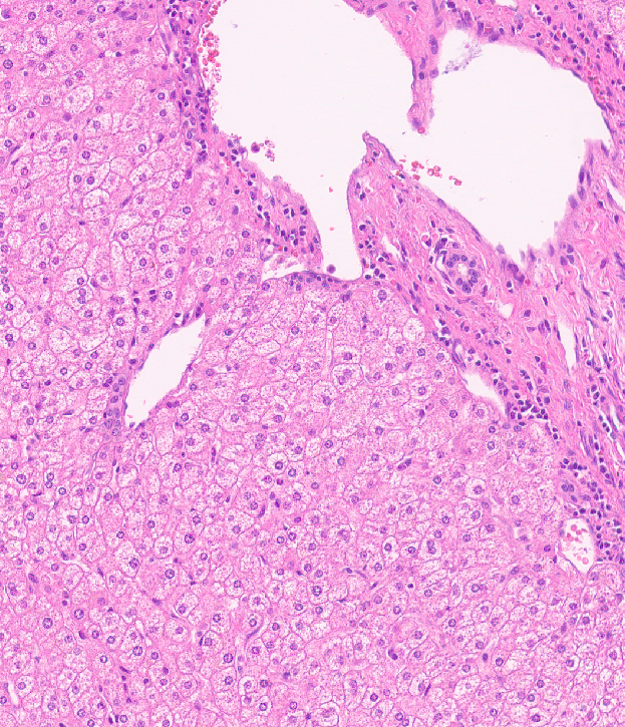
Fig 10
Pathologic findings
There is architectural alteration, with parenchymal nodularity and altered portal and central spacing, but no cirrhosis. There are thin fibrous septa, some of which end blindly. (Figures 1 and 2). There is no significant inflammation (Figure 3). Fibrous spikes can be seen from portal tracts (Figure 4). Perforated and split septa (Figure 5) with hepatocytic “buds” (Figure 6), and isolated thick collagen bundles within the parenchyma (Figures 6 and 7) are seen. These features are part of the “hepatic repair complex” (see discussion). Vascular aberrations are noted in both portal and central areas. Remnant, small portal tracts with absent portal veins are noted (Figure 8) and some wide, ectopic vascular channels are present (Figure 9) suggestive of shunts. Vascular channels in some fibrous septa (Figure 10) may represent portal-venous connections without intervening sinusoids. The findings on special stains are discussed below.
Diagnosis
Regressed cirrhosis/ incomplete septal cirrhosis, history of treated hepatitis C
Discussion
End stage chronic liver disease from any underlying cause may result in advanced fibrosis, and eventually undergo parenchymal and vascular remodelling known as cirrhosis. At this stage, there are manifestations of portal hypertension and a high risk for development of hepatocellular carcinoma.
However, fibrosis can regress at any stage of liver disease, including in cirrhosis, and for any underlying etiology, in whom the underlying cause of liver damage is adequately treated. Resorption of fibrous septa occurs with regression, but the vascular alterations usually do not reverse, and some nodularity may persist. Histologic improvement may result in clinical improvement, manifested by improvement in hepatic function. However, as illustrated in our case, because of the irreversibility of vascular remodeling, there is usually a persistence of portal hypertension in incomplete septal cirrhosis/ regressed cirrhosis.
The current understanding of the underlying pathologic mechanisms involves a variably dynamic interplay of fibrogenesis and fibrolysis depending on the degree of insult: the net architectural alteration reflects a balance of injury and repair. As regression of fibrosis starts dominating, fibrous septa lose inflammatory infiltrate and edema, and there is resorption of some of the collagen. Consequently, the septa become thin and delicate. With regeneration of hepatocytes, the septa may be perforated or fragmented, and also leads to split septa or blind ending septa. Portal tract remnants can now be recognized which were earlier incorporated within dense circumferential fibrous bands. Scant fibrous stroma holds paired artery/ arteriole next to bile duct, but small portal veins are often obliterated. Recanalized large hepatic veins can be seen, but fibroelastotic cords representing occlusion of original hepatic vein branches can also be identified. Isolated thick collagen fibers within the parenchyma result from undigested remnants of fibrous septa.
The commonly used histologic scoring systems for fibrosis were developed before effective therapies became available, therefore fibrosis was only considered to be progressive. The Knodell, Ishak, Batts-Ludwig, Laennec, and Metavir staging systems show a unidirectional development of collagen deposition involving the hepatic parenchyma. None of these adequately capture the abnormal fibrosis or remodeling of regression of cirrhosis. The more recent Beijing classification attempts to fill this gap by classifying the pattern of fibrosis into 3 subcategories: progressive, indeterminant, and regressive. The microscopic features termed “the hepatic repair complex” (originally defined by Wanless et al) are included for the latter category.
Interestingly, the risk of hepatocellular carcinoma does not regress with regression of fibrosis/cirrhosis. Driver mutations already imparted early in the disease persist. Moreover, repeated cycles of injury and repair may result in genomic instability. On the other hand, appropriate treatment of the inciting agent at any step may prevent or delay further genetic/ epigenetic alterations. The actual risk assessment in this situation is unknown, but a lifelong surveillance for development of hepatocellular malignancy is currently advised. This has been well demonstrated in not only treated chronic viral hepatitis B and C, but also in phelobotomized hereditary hemochromatosis patients.
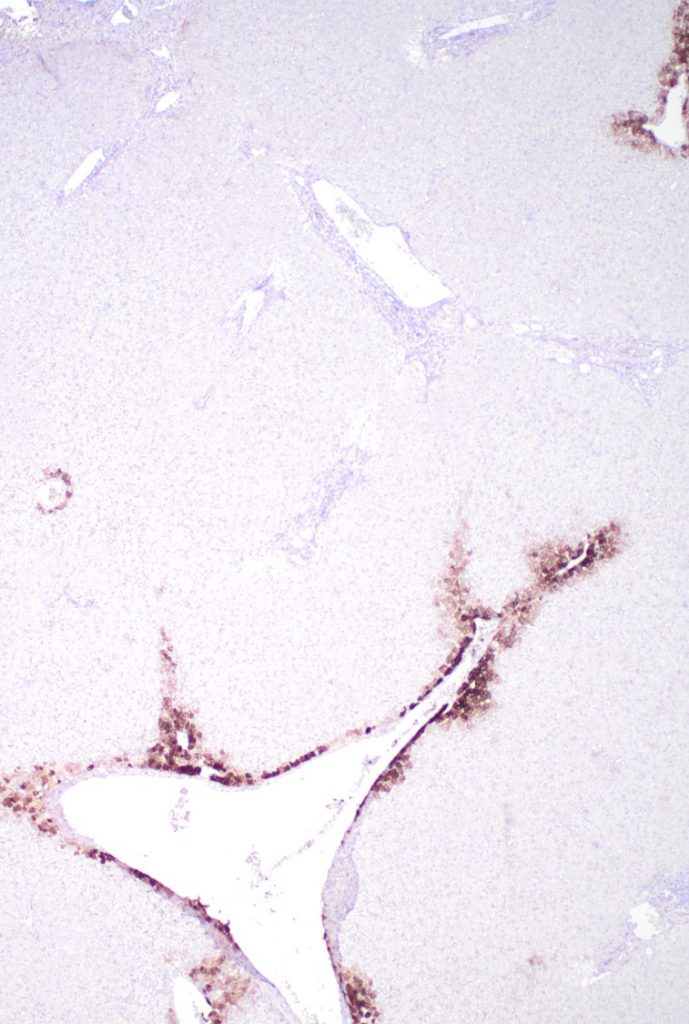
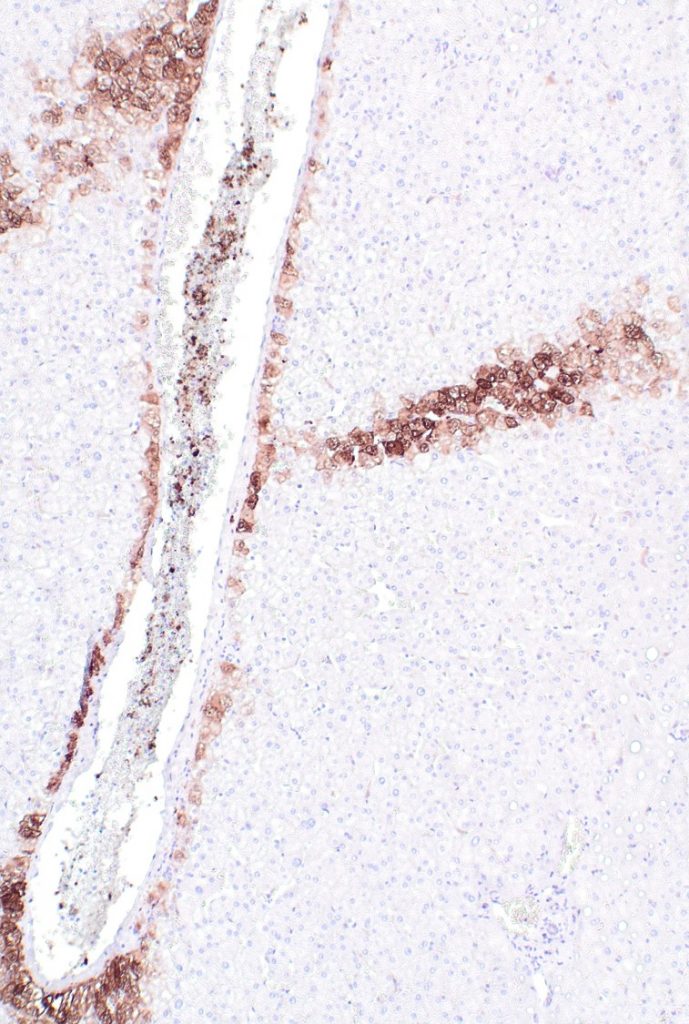
Special and immunohistochemical stain findings: Although several features of hepatic repair complex are noticeable on H&E stain, trichrome and reticulin stains will confirm these findings. CD34 will demonstrate as abnormally expanded expression (capillarization) involving the sinusoids (Figure 11), and glutamine synthase stain (highlighting perivenular hepatocytes) may be helpful to delineate the original location of central vein as well as highlight recanalized hepatic veins (Figure 12 A), and demonstrate the degree of architectural alteration with marked variation in portal-central spacing (Figure 12 B).
In this particular case, the patient had successfully completed treatment for Hepatitis C. She was on routine surveillance for the inherent risk of development of hepatocellular carcinoma, and the tumor was detected at a relatively early stage (original tumor size 1.8 cm). After 5 years of excision of the primary tumor, we examined her liver on the partial hepatectomy that we received for a recurrence of the tumor. Per intraoperative note, there was no nodularity, yet, the abnormal fibrosis and altered vascularity is readily apparent on microscopic examination. Several of the histologic features defined by hepatic repair complex are identified in this case, therefore the diagnosis is consistent with incomplete septal cirrhosis/ regressed cirrhosis. Interestingly, although there were no transjugular venous pressure measurements performed at this institution, her low platelets are an indication of underlying portal hypertension, that is known to be irreversible in this setting. The phenomenon of regression of advanced fibrosis with successful treatment of the underlying etiology but persistence of microvascular alterations is demonstrated through this case.
Special note: Although significant understanding and acceptance of the concept of regressed cirrhosis has taken place in the last two decades, Hans Popper, one of the founders of hepatopathology, whose name and existence are integral to this society, was one of the earliest proponents of the reversal of fibrosis (dating back to early 1960s). He also coined the term “incomplete septal cirrhosis”, to the vaguely macronodular appearance of the parenchyma, delineated by delicate, incomplete septa.
Fig 11. CD34
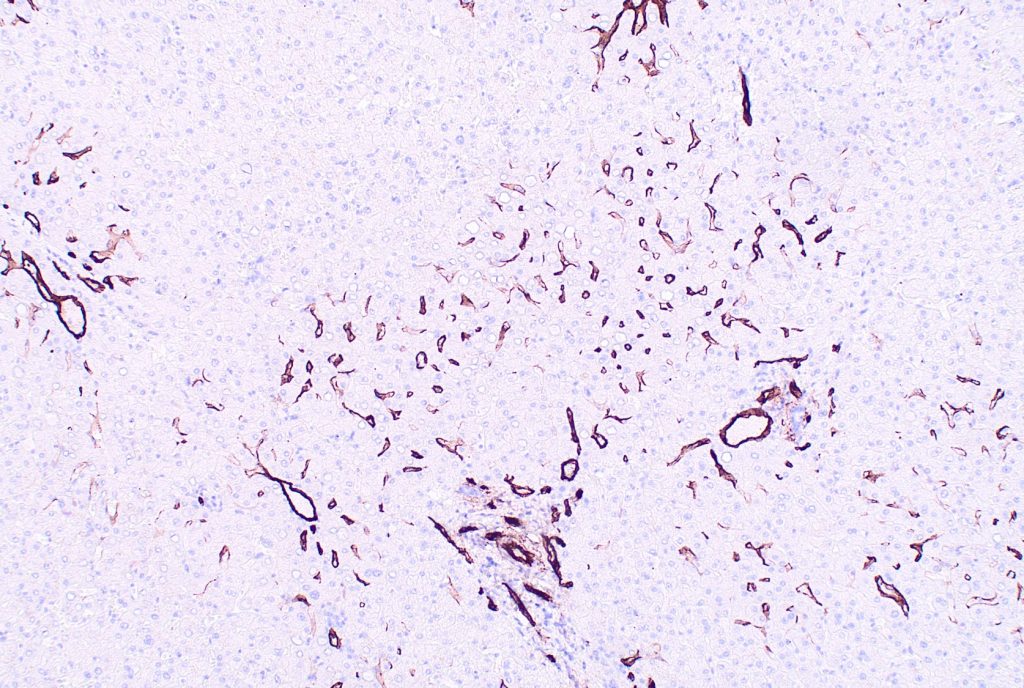
Fig 12A. Glutamine synthase Immunostain
12B
References
Khan S, Saxena R. Regression of Hepatic Fibrosis and Evolution of Cirrhosis: A Concise Review. Adv Anat Pathol. 2021 Nov 1;28(6):408-414.
Hytiroglou P, Theise ND. Regression of human cirrhosis: an update, 18 years after the pioneering article by Wanless et al. Virchows Arch. 2018 Jul;473(1):15-22.
Theise ND, Jia J, Sun Y, Wee A, You H. Progression and regression of fibrosis in viral hepatitis in the treatment era: the Beijing classification. Mod Pathol. 2018 Aug;31(8):1191-1200.
Wanless IR, Nakashima E, Sherman M (2000) Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med 124:1599–1607.
Sun Y, Zhou J, Wang L, Wu X, Chen Y, Piao H, Lu L, Jiang W, Xu Y, Feng B, Nan Y, Xie W, Chen G, Zheng H, Li H, Ding H, Liu H, Lv F, Shao C, Wang T, Ou X, Wang B, Chen S, Wee A, Theise ND, You H, Jia J (2017) New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment. Hepatology 65:1438–1450.
Hutterer F, Rubin E, Popper H. Mechanism of collagen resorption in reversible hepatic fibrosis. Exp Mol Pathol. 1964;3:215–223.
Dr. Chatterjee would like to thank Dr. EM Brunt for her valuable help.
