Interesting Case April 2021
Case contributed by:
Juhi Mahadik, Natalia Golardi, Reka Szigeti, Shilpa Jain
Baylor College of Medicine, Houston
Have an interesting case to share?
Contact HPHS newsletter committee chair Sadhna.Dhingra@ProPath.com
Case history
A 32 year old Hispanic female with primary vitreoretinal B-cell lymphoma with CNS involvement diagnosed at outside hospital presented with worsening transaminitis. She had recently received first cycle of chemotherapy (methotrexate, cytarabine, thiotepa, rituximab) and intravitreal methotrexate. Her transaminases were almost normal till a month before the liver biopsy; at the time of biopsy the ALT was 346 U/L, AST- 234U/L, ALP- 544 U/L, total bilirubin- 0.4 mg/dl. ANA, AMA, ASMA were all negative. Peripheral blood and bone marrow were negative for involvement by lymphoma by morphology and flow cytometry (WBC- 6.4/uL, Hb- 11.1 g/dl, Plt- 413/uL).
Viral hepatitis markers were negative. However, her EBV IgG was high (>600 U/ml) while IgM was negative against the viral capsid antigen (VCA) with EBV DNA of 27065 copies/ml. Increasing transaminitis led to liver biopsy with a concern for chemotherapy/drug induced liver injury, EBV reactivation versus involvement by lymphoma.
Liver biopsy images
Figure A: Infiltration of hepatic sinusoids by lymphocytes with no cytological atypia (H&E, 400x magnification)
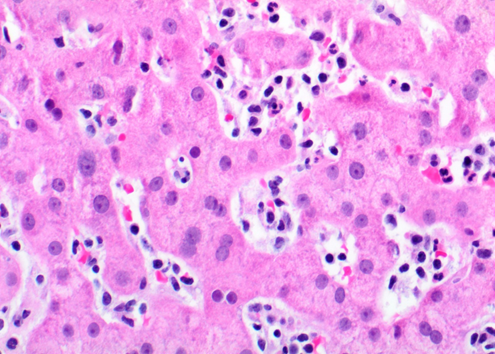
Figure b: Area of spotty necrosis of hepatocytes (H&E, 200x magnification)
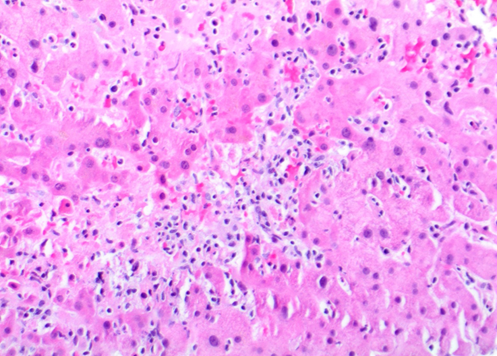
Figure c: Focal venulitis (H&E, 200x magnification)
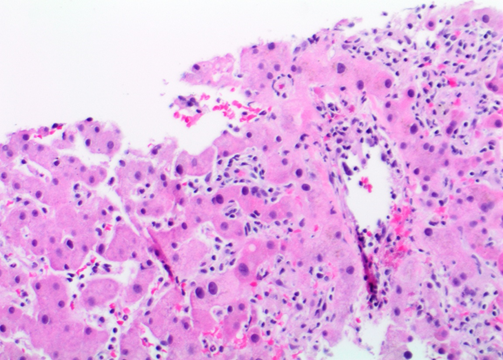
Figure d: Lymphocytes positive for EBER by in-situ hybridization (200x magnification)
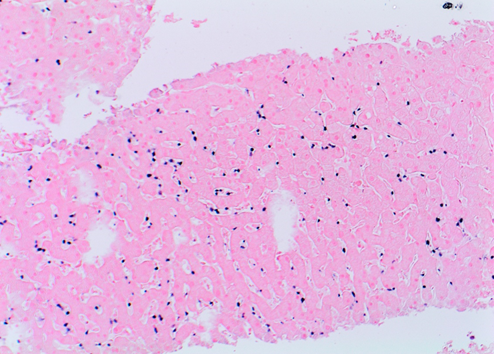
Figure e: Negative CD20 in lymphocytes (100x magnification)
Figure f: Lymphocytes positive for CD3 (200x magnification)
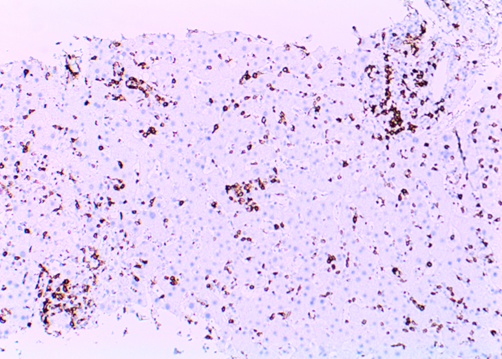
Figure g: Lymphocytes positive for CD8 (200x magnification)
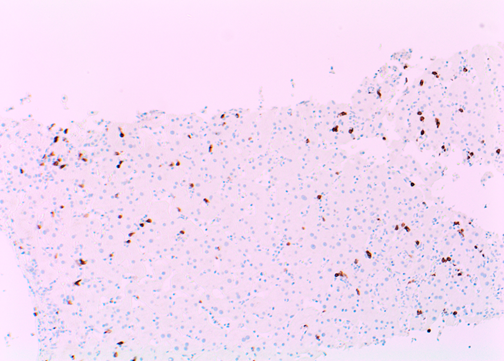
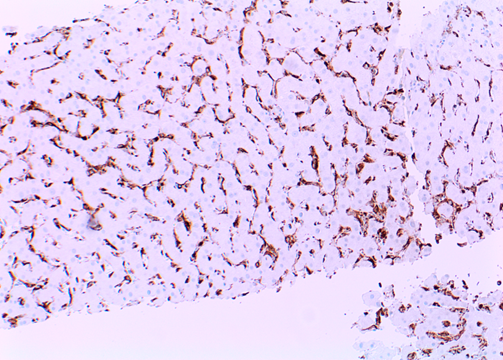
Figure h: Macrophages staining with CD68 in hepatic sinusoids (200x magnification)
Pathology findings
Liver biopsy showed an acute hepatitis pattern with diffuse infiltration of hepatic sinusoids by lymphocytes (Fig. A) with spotty necrosis (Fig. B). Portal triads showed lymphohistiocytic infiltrate with no cytological atypia, eosinophils and focal venulitis (Fig. C). Bile ducts were preserved. In-situ hybridization (ISH) for EBV encoded RNA (EBER) was positive in lymphocytes in the sinusoids (Fig. D). These lymphocytes were further characterised and were positive for CD3 (T-cell) (Fig. E), CD8 (Fig. F) and negative for PAX-5 and CD20 (B-cell markers) (Fig G). They and negative for CD4, CD56 (NK-cell) and CD34 (immature hematopoietic cell) markers. CD68 highlighted diffuse sinusoidal hyperplasia but no significant hemophagocytosis (Fig. H).
Diagnosis
Chronic Active Epstein-Barr virus infection of T- and NK-cell type: Acute Hepatitic presentation of an enigmatic disease
Discussion
EBV is known to infect oropharyngeal epithelial cells and B-lymphocytes during primary infection. While most such cases are asymptomatic in children, adolescents and young adults often develop fever, tonsillitis and cervical lymphadenopathy, characteristic of IM [1]. A strong T-cell response develops against the infected B-cells in IM, which helps in controlling the infection [2]. The virus then establishes a persistent infection of host B-cells by switching to a latent phase with expression of several latent membrane proteins (LMP1, LMP2A, LMP2B) and EBV nuclear antigens (EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C) [3]. Viral reactivation can occur whenever there is an impairment of the cell-mediated immunity and lead to malignant disorders like Burkitt lymphoma, diffuse large B-cell lymphoma, Hodgkin lymphoma, extranodal NK/T-cell lymphoma-nasal type, aggressive NK-cell leukemia and nasopharyngeal carcinoma [3,4]. Whereas the receptor for entry of EBV into B-cells is widely known to be CD21, how the virus infects T-cells, NK-cells and macrophages, is less well explored.
In some patients, especially those of East Asian ethnicity and Latin America, EBV can cause a chronic infection of T- and NK-cells and lead to prolonged manifestations. According to the most recent World Health Organization Classification of tumors of hematopoietic and lymphoid tissues [5], chronic active EBV infection of T- and NK-cell type is included within the disease spectrum of EBV-positive T- and NK-cell lymphoproliferative diseases of childhood. It can present either in a systemic or a localized cutaneous form with a progression to overt lymphoma occurring in a few cases, over varying periods of time. CAEBV infection of B-cells also occurs and has been found more often in patients from North America [6].
CAEBV of T- and NK-cell type, systemic form is diagnosed with features of IM-like symptoms for >3 months, increased EBV DNA (>102.5 copies/ml) in the peripheral blood and histologic evidence of infiltration of the affected organs by EBV positive cells. Although originally thought to be a disease of childhood, now it is known that adults can also be affected. Liver, spleen, lymph nodes, bone marrow and skin are commonly involved [5]. Fever, hepatosplenomegaly and lymphadenopathy are the most common symptoms but some patients also report skin eruptions, uveitis and diarrhea [4,5]. Although an absence of apparent immunodeficiency has been underscored in the diagnostic criteria, several patients have been shown to have reduced precursors of EBV-specific cytotoxic T-lymphocytes [6]. The strong racial predisposition is further suggestive of minor cellular immunodeficiencies or genetic polymorphisms in genes related to the EBV immune response. Patients with NK-cell type disease fare better in terms of survival than those with T-cell type [5]. Studies have shown promising results only for hematopoietic stem cell transplant as a curative form of therapy. Antivirals, chemotherapy and immunomodulatory agents have been tried but are not effective [7].
Our patient had a persistently high VCA IgG for a year, high EBV DNA and multiple episodes of transaminitis. EBV DNA was done only after she started chemotherapy, so it remained unclear for how long the viral proliferation had been going on, as CAEBV generally affects immunocompetent patients. EBV reactivation hepatitis was another diagnostic consideration, due to her immunocompromised state. EBV hepatitis also shows a diffuse sinusoidal infiltrate by lymphocytes in a “string of beads” pattern. The lymphocytes consist primarily of reactive T-cells, NK cells and rare EBV-infected B-cells [1,2]. EBV infection of B-cells can be demonstrated by EBER ISH. In our patient, the lymphocytes were mostly CD8 positive T-cells that showed EBER positivity by ISH. A similar infiltration of sinusoids by lymphoid cells can be seen in lymphoproliferative disorders affecting the liver, such as hepatosplenic T-cell lymphoma, adult human T-cell leukemia, natural killer cell leukemia and others [1]. Absence of atypia in the lymphoid cells along with EBER positivity pointed against a lymphoproliferative process. Furthermore, clonality studies may not always help in the distinction as CAEBV can be oligoclonal, polyclonal or even monoclonal [5,6]. To summarize, the diffuse sinusoidal infiltration by CD3, CD8, EBER positive T-cells, presence of high viral DNA copies in the peripheral blood and the histologic evidence of liver injury, was a strong evidence for a CAEBV with liver involvement.
Learning points:
- A high index of suspicion is necessary for an accurate diagnosis of CAEBV of T- and NK-cell type, which although rare, can certainly occur and confound the clinical picture in patients from particular ethnicities.
- In absence of other viral hepatitis markers, checking for EBV serology and viral load can be helpful, especially when the histology is as described above.
- Further characterization of the lymphoid infiltrate into B-, T- or NK-cells should be done as that has prognostic implications.
References
- Schechter S, Lamps L. Epstein-Barr Virus Hepatitis: A Review of Clinicopathologic Features and Differential Diagnosis. Arch Pathol Lab Med. 2018 Oct;142(10):1191-1195.
- Negro F. The paradox of Epstein-Barr virus-associated hepatitis. J Hepatol. 2006 May;44(5):839-41. doi: 10.1016/j.jhep.2006.03.002. Epub 2006 Mar 10. PMID: 16554104.
- Kerr JR. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol. 2019 Oct;72(10):651-658. doi: 10.1136/jclinpath-2019-205822. Epub 2019 Jul 17. PMID: 31315893.
- Kimura H, Fujiwara S. Overview of EBV-Associated T/NK-Cell Lymphoproliferative Diseases. Front Pediatr. 2019 Jan 4;6:417.
- Quintanilla-Martinez L., Ko Y.H., Kimura H., Jaffe E.S. EBV-positive T-cell and NK-cell lymphoproliferative diseases of childhood. In: Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., Arber D.A., Hasserjian R.P., Le Beau M.M., et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2017. pp. 355–362.
- Fujiwara S, Nakamura H. Chronic Active Epstein-Barr Virus Infection: Is It Immunodeficiency, Malignancy, or Both?. Cancers (Basel). 2020;12(11):3202.
- Cohen JI, Jaffe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117(22):5835-5849.
