Interesting Case January 2021
Case contributed by:
Till S. Clauditz MD, Dept. of Pathology University-Medical Center Hamburg-Eppendorf, Germany
Till Krech MD, PathoCom, Osnabrueck, Germany
Have an interesting case to share?
Contact HPHS newsletter committee chair Sadhna.Dhingra@ProPath.com
Clinical history
The patient is a 73-year old man with a complicated medical history including prostate carcinoma (Gleason 3+5=8, PSA 12.1 ng/ml), squamous cell carcinoma of the skin, obesity, diabetes mellitus type II and non-alcoholic fatty liver disease (NAFLD). During tumor staging, a solitary round focus was found in the liver (1.8 cm; MRI), which was surgically resected and assumed to a metastasis of the prostate carcinoma. Liver enzymes prior to surgery were elevated (AST 298, ALT 304, GGT 19, AP70). Post-surgery, the patient showed signs of hydropic decompensation with ascites and developed an infected bilioma.
Pathologic findings
Macroscopically, a solitary liver lesion with a diameter of 1.5 cm was noted. Histology showed an infiltrative tubular neoplasm with anastomosing glands and with areas of cystic dilatation, as well as small solitary tubules with an accompanying lymphatic infiltrate (Fig. 1 + 2). Parts of the tumor showed entrapped single or clusters of hepatocytes (Fig. 3). The tumor cells were mainly cuboidal with sparse eosinophilic cytoplasm. The nuclei were round to oval with chromatin clearing and small nucleoli (Fig. 4). Immunohistochemical workup showed positivity for CK7 (Fig. 5), weak nuclear p53 staining (wild type expression) and negativity for: CK19, Prostate-Specific Membrane Antigen (PSMA), IMP3, S100p, p16 and CD56. In-situ hybridization for albumin was positive (Fig. 6). The non-neoplastic liver parenchyma showed mild macrovesicular steatosis with stage 2 fibrosis (according to Kleiner et al.; 1) consistent with NAFLD (not shown).
Figure 1
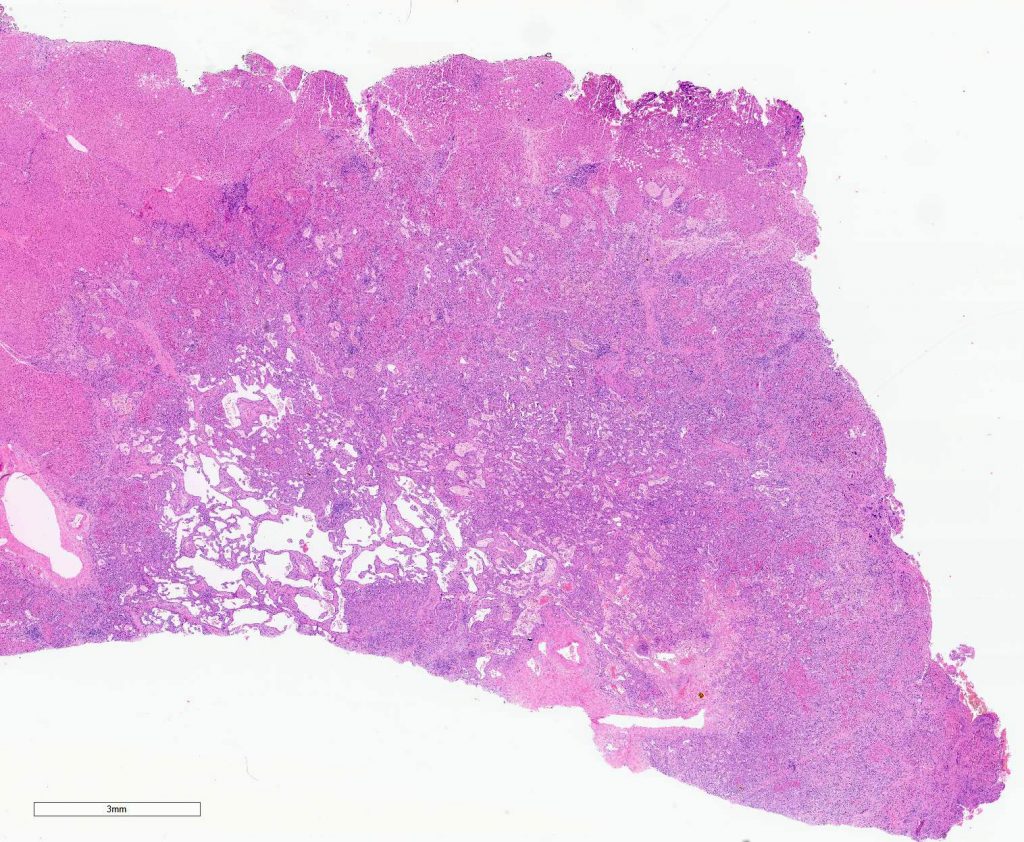
Figure 2
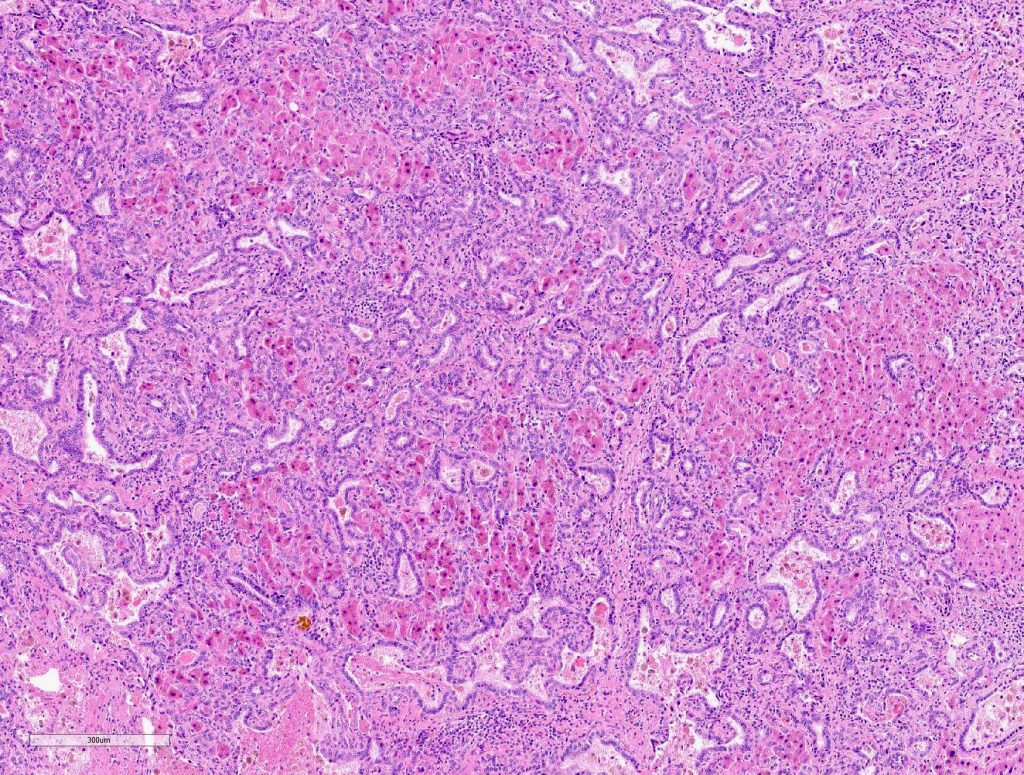
Figure 3
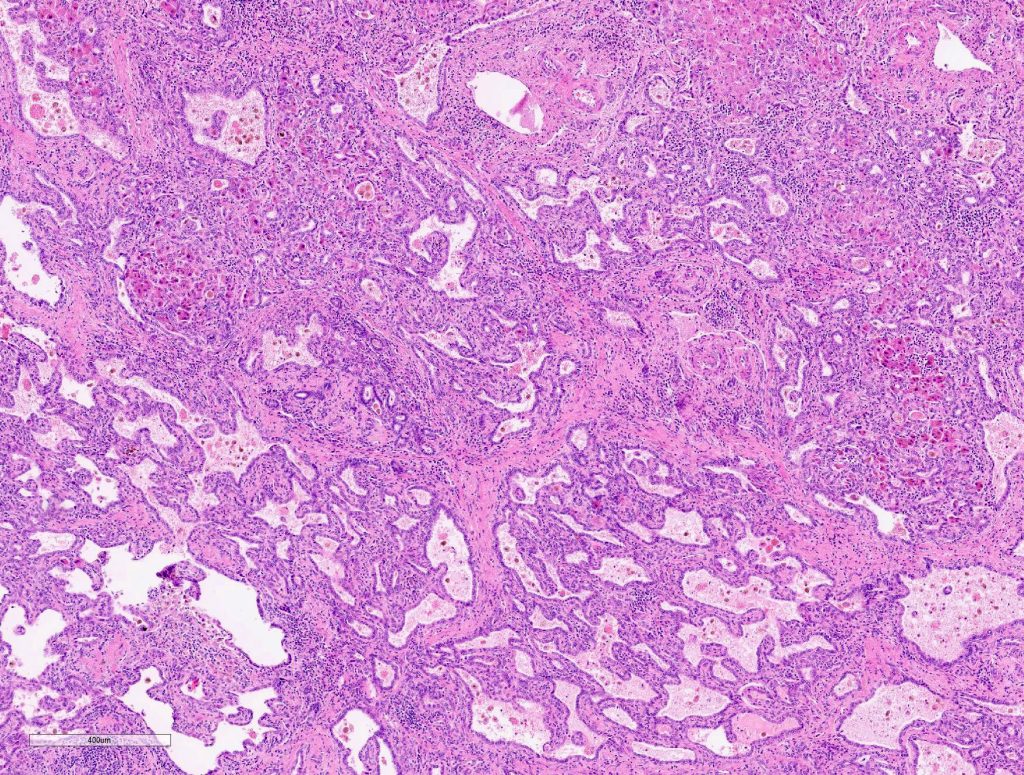
Figure 4
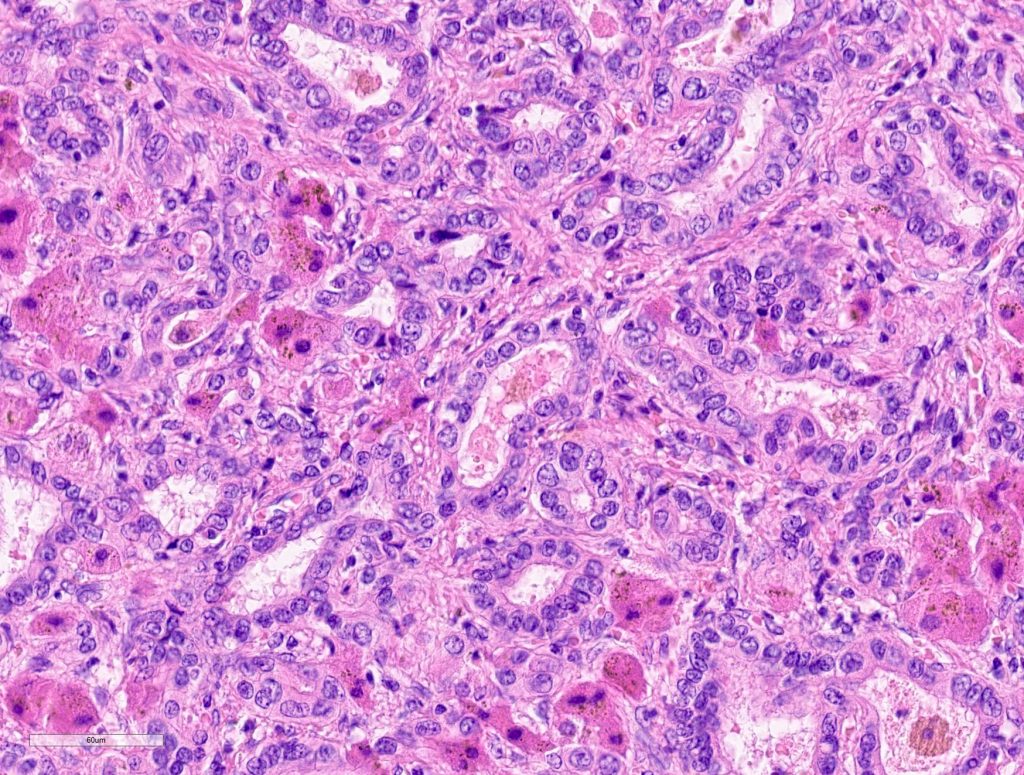
Figure 5
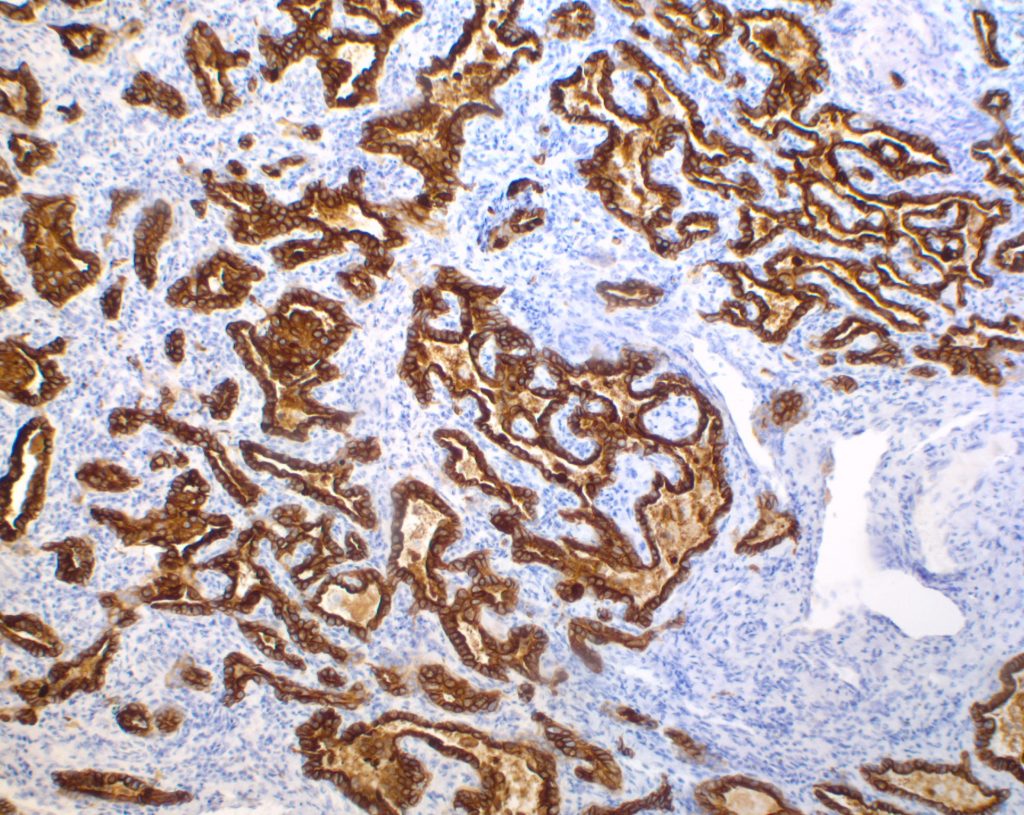
Figure 6
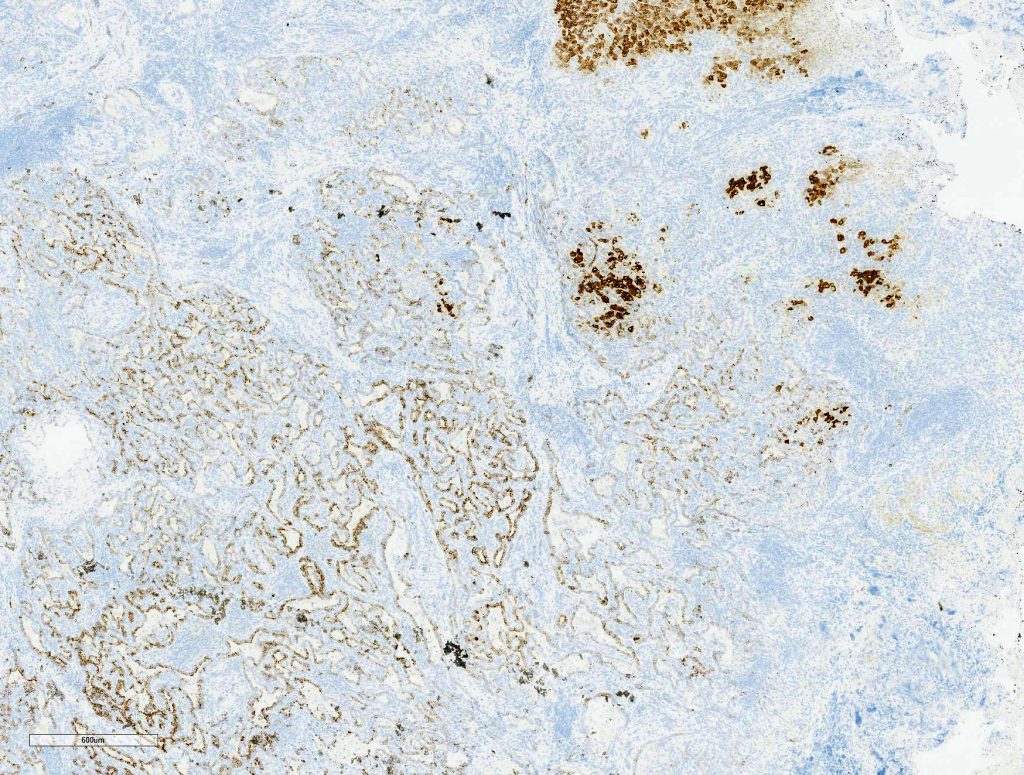
Diagnosis
Cholangiocarcinoma, ductal plate malformation type (CC with DMT pattern)
Discussion
Intrahepatic Cholangiocarcinoma (CC) is a primary malignant liver tumor characterized by cholangiocytic differentiation (2). CC shows broad histologic diversity which may reflect its origin of topographically heterogenous cholangiocytes in the biliary tree (3). Two main types of CC with distinct clinical, pathologic and molecular features can be distinguished: large duct type carcinomas and small duct type carcinomas. Whereas large duct type carcinomas comprise a group with classical features of adenocarcinomas including obvious mucin production, small duct type carcinomas can look quite inconspicuous and typically lack mucin production (2). One peculiar example of a small duct carcinoma is the cholangiolocellular carcinoma (CLC), which resembles a reactive ductular proliferation (4). CC with predominant ductal plate malformation pattern is a rare subtype of CC which was first described by Nakanuma et al in 2012 (5). Most of the previously described cases were associated with substantial areas of cholangiolocellular carcinoma (5, 10). Based on the morphologic overlap and genetic similarities CC with ductal plate malformation pattern may be regarded as a subtype of CLC (4,5, 10).
The ductal plate is a biliary structure which can be found in the fetal liver and usually disappears during the maturation of the biliary tree. Fibropolycystic liver diseases such as polycystic kidney and liver disease, congenital hepatic fibrosis and Caroli disease are characterized by malformations of the ductal plate. A ductal plate remnant most pathologists are probably familiar with is the von-Meyenburg complex or biliary microhamartoma (6). A recent study by Bhalla et al. reported of von Meyenburg complex associated carcinomas (11). If these neoplasms have morphologic and genetic similarities with CC with DPM pattern needs to be evaluated in further studies.
Intrahepatic CC with predominant ductal plate malformation pattern shows dilated irregular, staghorn shaped ductal structures closely resembling von-Meyenburg complexes and thus making it a challenging diagnosis especially in biopsy samples. To differentiate hamartomatous changes from CC with DPM pattern in liver biopsies entrapped single or clusters of hepatocytes might be a histomorphological finding, which might be helpful. Like other small duct type carcinomas CC with DPM pattern may stain for CD56 (NCAM), although this is of limited diagnostic value as reactive ductular proliferations also express this marker (2, 5, 6). The hepatic origin of the tumor infiltrates was confirmed by a positive albumin in-situ hybridization (ISH), described by Brackett et al. as a reliable method to determine hepatic origin in cases of hepato- and cholangiocellular carcinoma (9).
Otherwise well-established immunohistochemial stains for the diagnosis of CC such as S100p, IMP3, p53 and p16 do not seem to be helpful for the diagnosis of CC with DPM pattern (7, 8, 12). According to our results and the findings by Komuta et al. S100p, as well as IMP3 are not expressed by CC with predominant DPM pattern. Futhermore, Nakanuma et al reported p53 being expressed only scattered in the tumor cells, as seen in our case (5). The most recent study on CC with predominant DPM pattern by Sasaki et al showed ARID1A genetic alterations with consecutive loss of ARID1A expression making it a promising candidate for diagnostic immunohistochemical evaluation in the future (10).
Learning points
- Because of its morphologic similarities with bile duct hamartoma and ductal plate malformation diagnosis of CC with DMT pattern might be challenging and even impossible in liver biopsy specimen.
- In challenging cases, especially in biopsy specimens, entrapped single or clusters of hepatocytes might be helpful clues to make the diagnosis
- Albumin in-situ hybridization establishes hepatic origin, as reported for “conventional” cholangiocarcinoma
- ARID1A expression might be a diagnostic tool in the diagnosis of CC with DPM pattern
References
1. D.E. Kleiner et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease; Hepatology, 41 (2005), pp. 1313-1321
2. WHO Classification of Tumors Editorial Board. Digestive System Tumors. Lyon (France): International Agency for Research on Cancer; 2019. (WHO classification of tumors series; 5th ed.; vol 1)
3 . Komuta M et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012 Jun;55(6):1876-88. doi: 10.1002/hep.25595. PMID: 22271564
4. Torbenson M, Zen Y, Yeh MM. Tumors of the liver, AFIP Atlas of tumor pathology series 4. Washington (DC): American Registry of Pathology 2018.
5. Nakanuma Y et al. Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: a new subtype. Am J Surg Pathol. 2012 Nov;36(11):1629-35. doi: 10.1097/PAS.0b013e31826e0249. PMID: 23073321
6. Burt, Ferrell, Hubscher. MacSween’s Pathology of the Liver; 7th Ed., Elsevier, July 2017
7. Riener MO et al. IMP3 expression in lesions of the biliary tract: a marker for high-grade dysplasia and an independent prognostic factor in bile duct carcinomas. Hum Pathol. 2009 Oct;40(10):1377-83. doi: 10.1016/j.humpath.2009.01.024. Epub 2009 May 20. PMID: 19467694
8 Shin H et al. Calcium-binding protein S100P is a novel diagnostic marker of cholangiocarcinoma. Cancer Sci. 2011 Jan;102(1):150-6. doi: 10.1111/j.1349-7006.2010.01757.x. Epub 2010 Oct 13. PMID: 21029254
9. Brackett DG t al. Cholangiolar pattern and albumin in situ hybridisation enable a diagnosis of intrahepatic cholangiocarcinoma. J Clin Pathol. 2020 Jan;73(1):23-29. doi: 10.1136/jclinpath-2019-206055. Epub 2019 Aug 17.PMID: 31422372
10. Sasaki M et al. Cholangiolocellular Carcinoma With “Ductal Plate Malformation” Pattern May Be Characterized by ARID1A Genetic Alterations. Am J Surg Pathol. 2019 Mar;43(3):352-360. doi: 10.1097/PAS.0000000000001201. PMID: 30520820
11. Bhalla A. et al. Histopathological evidence of neoplastic progression of von Meyenburg complex to intrahepatic cholangiocarcinoma. Hum Pathol 2017, 67: 217-224. PMID: 28823571
12. Tannapfel A et al. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000 Nov;47(5):721-7. doi: 10.1136/gut.47.5.721. PMID: 11034592
