Interesting Case July 2020
Case contributed by:
Alison Chan, D.O. and Karen Matsukuma, M.D., Ph.D.
University of California Davis, Department of Pathology and Laboratory Medicine, Sacramento, CA
Have an interesting case to share?
Contact HPHS newsletter committee chair Sadhna.Dhingra@ProPath.com
Clinical History
A 55-year-old female with a history of endometrial cancer, status post total abdominal hysterectomy 6 years prior, presented with intermittent right-sided abdominal pain for one week. A CT abdomen/pelvis with contrast demonstrated a 7.3 cm liver mass with adjacent perihepatic hematoma. No radiologic evidence or history of chronic liver disease was noted. Laboratory investigation showed an unremarkable CBC, AST of 21 IU/L, ALT of 24 IU/L, and alkaline phosphatase of 75 IU/L. Serologies for Hepatitis B and C were negative. Tumor marker values were as follows: alpha-fetoprotein 816.5 ng/mL, CA-125 43.7 U/mL, CA 19-9 40.7 U/mL, and carcinoembryonic antigen 1.9 ng/mL. A liver biopsy was performed.
Pathologic Findings
Figure 1: Low power view of the liver biopsy.
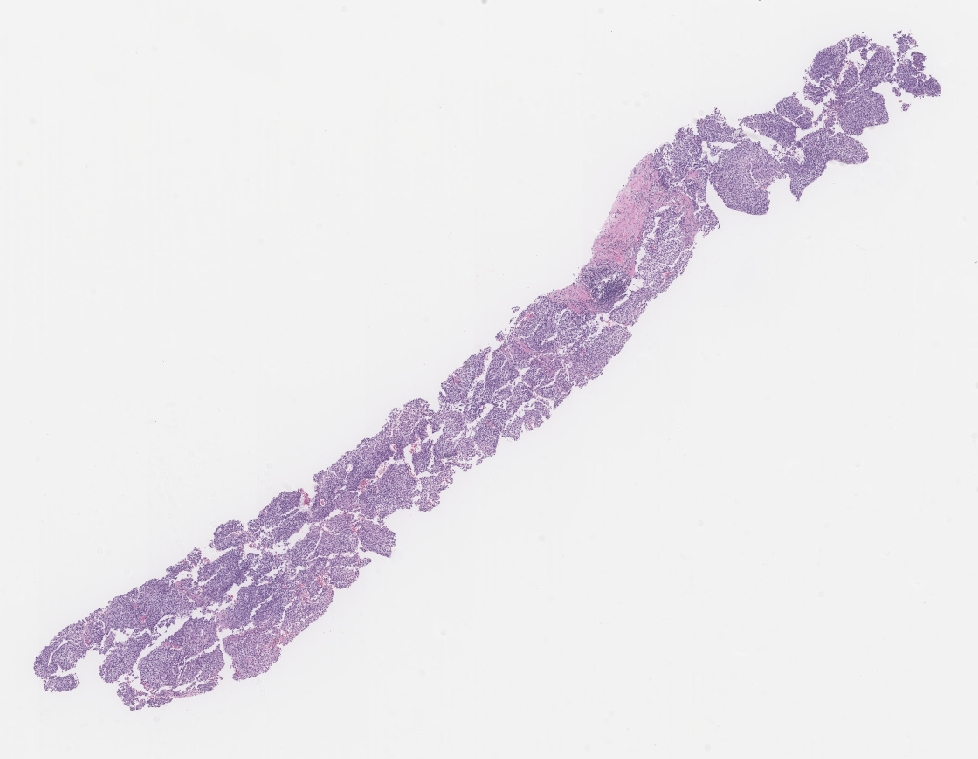
Figure 2: Medium power view of the liver biopsy shows a vaguely nodular sheet of monomorphic neoplastic cells, interrupted by slit-like vascular spaces.
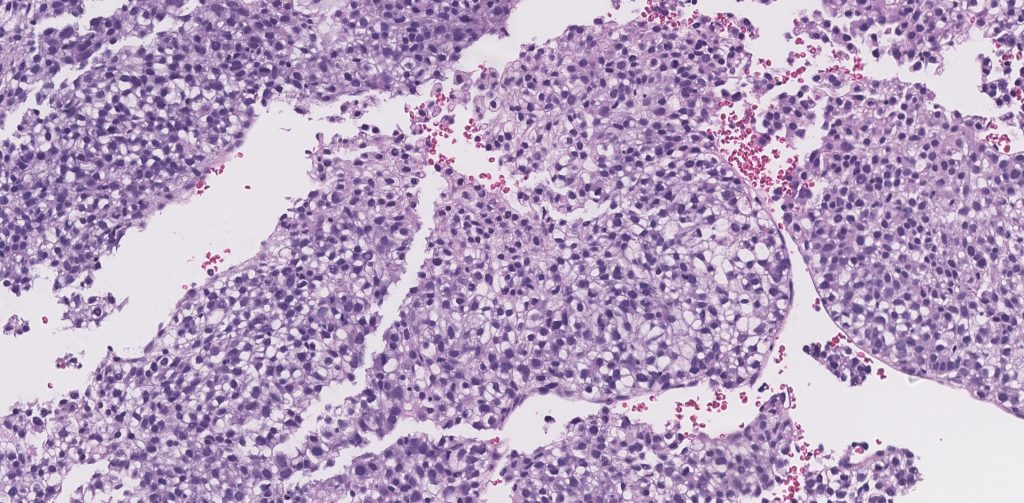
Figure 3: High power view of the liver biopsy shows relatively uniform tumor cells with round to oval nuclei and moderate to abundant clear cytoplasm.
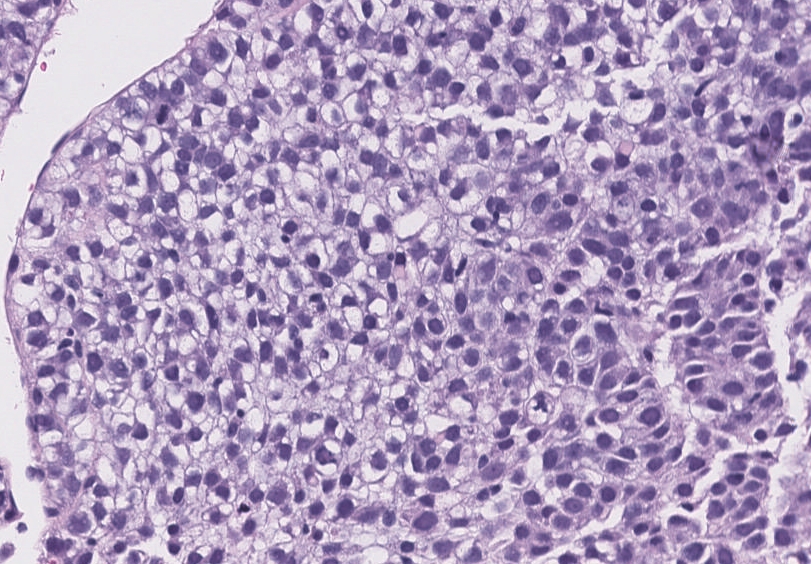
Figure 4: Immunohistochemical stains demonstrating focal expression of glypican-3 and CK7, and diffuse expression of AE1/AE3.
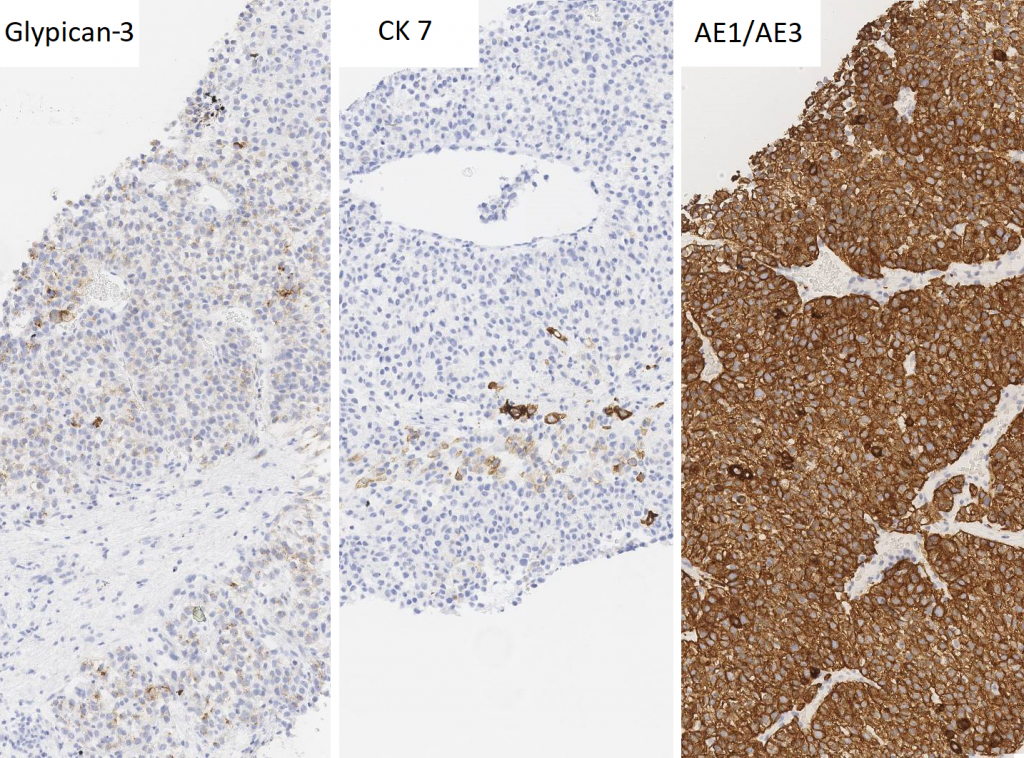
Biopsy Immunohistochemical Stains
AE1/AE3 Positive (diffuse, strong)
Glypican-3 Positive (focal)
Hep Par1 Negative
Arginase 1 Negative
CK7 Positive (focal)
PAX 8 Negative
ER Negative
PR Negative
Diagnosis:
Hepatocellular carcinoma, clear cell variant
Discussion:
Given the extremely high serum alpha-fetoprotein level and patchy positivity for glypican-3, the findings were deemed most consistent with hepatocellular carcinoma with clear cell features. Re-review of the arginase 1 stain also revealed very focal positivity. Hepatectomy was subsequently performed (Figure 5).
Figure 5: Low power view of the partial hepatectomy specimen shows juxtaposition of the clear cell component (right side) with areas demonstrating more conventional hepatocellular differentiation (left side).
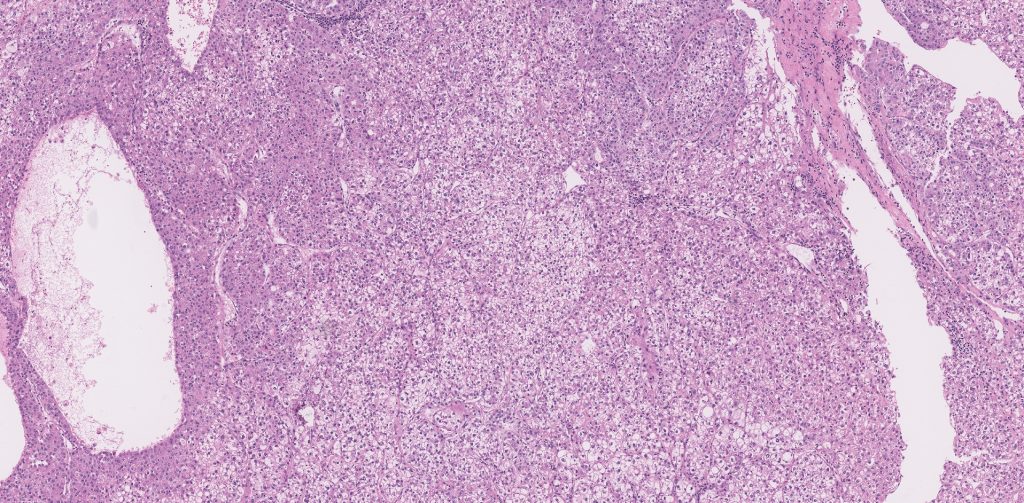
Figure 6: High power view of the optically clear cells of clear cell HCC.
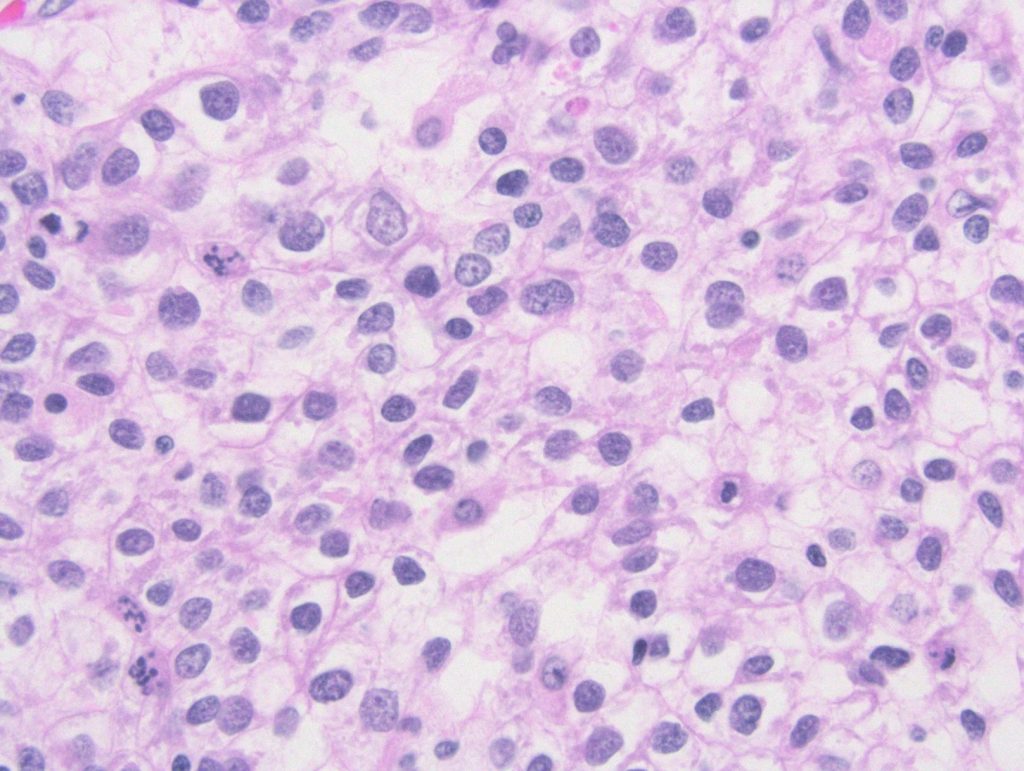
The clear cell variant of hepatocellular carcinoma (HCC) is one of the many subtypes of HCC, representing 3-7% of HCCs overall. The current World Health Organization criteria require at least 80% clear cell morphology for classification as clear cell HCC, whereas other observers require only 50% clear cell component. Interestingly, some studies show an association with Hepatitis C and better overall prognosis compared to classical HCC. Additionally, while many patients with clear cell HCC have cirrhosis, several studies highlight the existence of clear cell HCC in non-cirrhotic livers.
The defining histologic feature of clear cell HCC is the presence of tumor cells with large, optically clear cytoplasm due to accumulation of glycogen or, less commonly, lipid. The neoplastic cells can be arranged in trabecular, solid, and/or acinar patterns, and are not typically associated with significant intra-tumoral fibrosis.
Given the prominent intracytoplasmic accumulation of glycogen or lipid, clear cell HCC can demonstrate atypical imaging findings, and biopsy may be necessary to confirm the diagnosis. On abdominal ultrasound, increased echogenicity may be observed; on unenhanced CT there may be decreased attenuation; and on MRI, the tumor may be interpreted as having a fatty component. Consequently, the radiologic differential diagnosis may include hepatic adenoma (e.g., HNF1-inactivated subtype), angiomyolipoma, and other lipid-rich tumors.
When predominantly clear cell morphology is observed in a mass-directed liver biopsy (as in the current case), a wide differential diagnosis that includes primary and metastatic clear cell carcinomas should be considered. In addition to clear cell HCC, primary liver tumors such as clear cell variant of fibrolamellar HCC, classical HCC with foamy histiocytes, epithelioid angiomyolipoma, and clear cell cholangiocarcinoma are diagnostic possibilities. Metastatic carcinomas with clear cell morphology, including clear cell renal cell carcinoma, adrenal cortical carcinoma, clear cell adenocarcinomas of the salivary gland and female genitourinary tract, clear cell neuroendocrine tumors, yolk sac tumor, and metastases from other sites such as lung and thyroid are also important to consider. The co-existence of cytologic findings of classical HCC (e.g., abundant granular, eosinophilic cytoplasm, round, centrally placed nuclei with prominent nucleolus), intracytoplasmic or canalicular bile, Mallory-Denk bodies, and/or background chronic liver disease are clues to hepatic origin.
Also within the differential diagnosis are steatohepatitic variant
of HCC and HCC with steatosis. The steatohepatitic variant of HCC is
characterized by steatosis, ballooning degeneration, intra-tumoral
inflammation, Mallory-Denk bodies, and pericellular fibrosis (Figure 7),
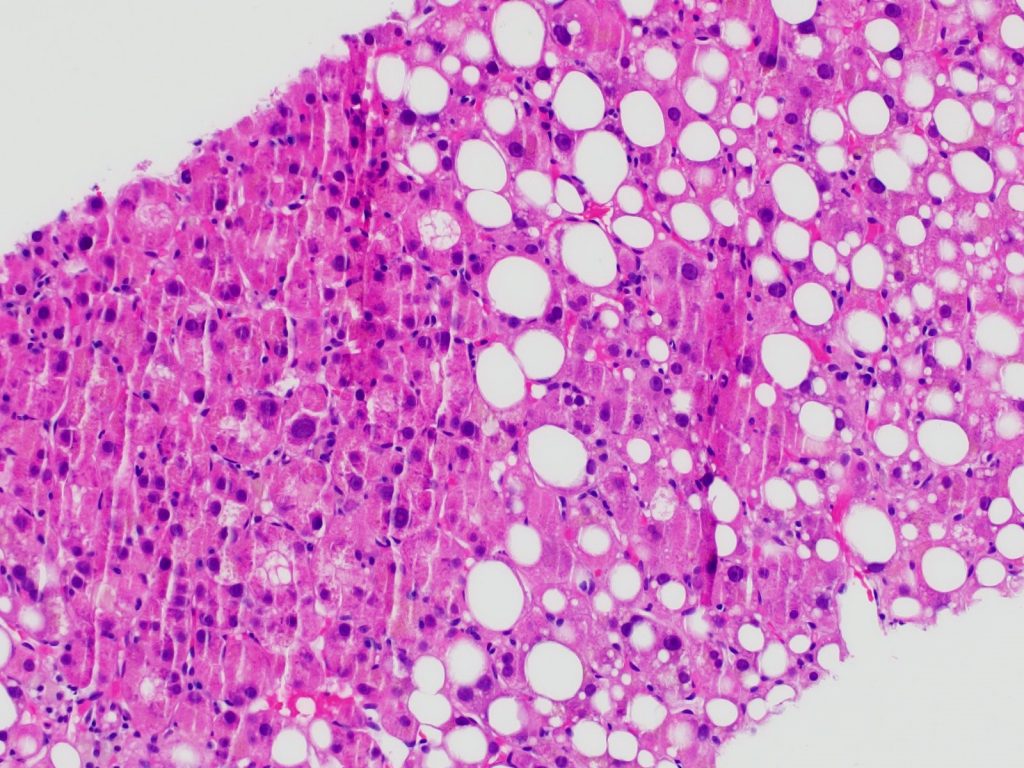
whereas HCC with steatosis contains foci of intra-tumoral steatosis but lacks
the hepatocyte ballooning, Mallory-Denk bodies, and inflammation (Figure 8). Although
in some instances clear cell HCC may be composed of neoplastic hepatocytes
containing tiny droplets of lipid resembling microvesicular steatosis (rather
than glycogen) or may contain distinct areas of fatty change, it will lack the
constellation of features that define steatohepatitis. Additionally, the vast
majority of steatohepatitic HCCs arise in a background of non-alcoholic fatty
liver disease or alcoholic liver disease. Thus, the presence or absence of a
hepatocyte injury pattern (e.g., ballooning degeneration, intra-tumoral
inflammation, pericellular fibrosis) and steatosis in the tumor and fatty liver
disease in the background liver will facilitate distinction of steatohepatitic
HCC from clear cell HCC. When necessary, PAS stain can aid in the distinction
of HCC with steatosis from clear cell HCC (when due to glycogen accumulation).
Figure 7: Steatohepatitic variant of HCC. Note the prominent ballooned hepatocytes (center), inflammation, Mallory-Denk bodies, and steatosis. Image courtesy of Sadhna Dhingra M.D.
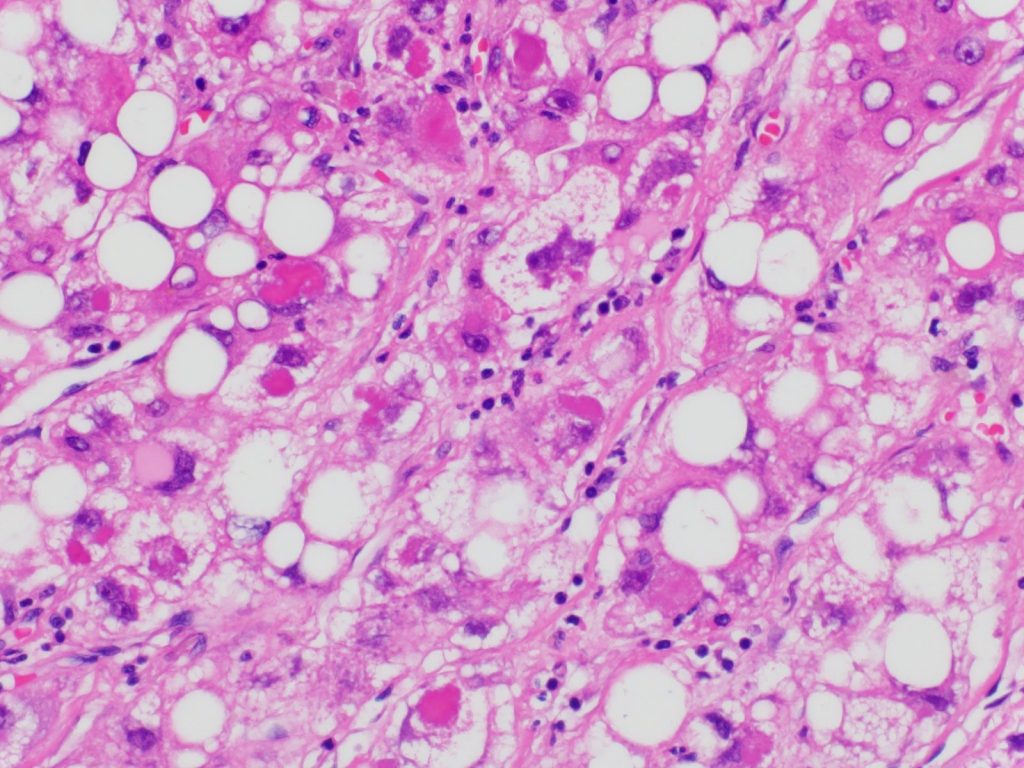
Figure 8: HCC with steatosis. Image courtesy of Sadhna Dhingra M.D.
In a subset of cases, immunohistochemistry is necessary to distinguish between the diagnostic possibilities. It is important to note that Hep Par1 expression can be patchy and variable in clear cell HCC (presumably due to displacement of normal cytoplasm by the abundant glycogen or lipid). Additionally, a subset of ovarian clear cell carcinomas expresses Hep Par1. Glypican-3, while occasionally mistaken as a specific marker of hepatocellular carcinoma, can be expressed in many tumors (e.g., yolk sac tumor, chromophobe renal cell carcinoma, lung carcinoma, cholangiocarcinoma, melanoma), and thus by itself is insufficient to confirm hepatocellular origin. CD10 and polyclonal CEA, other well-known markers of hepatocellular differentiation, can be positive in clear cell HCC but are similarly insufficient for confirming liver origin without other supportive features. In our case, because of the patient’s history of uterine cancer, markers of gynecologic carcinoma were performed (PAX8, ER, PR), all of which were negative. Interestingly, PAX8 may be positive in up to 20% of clear cell HCCs – adding to the list of immunohistochemical caveats for this tumor. Finally, although HCCs (including clear cell HCC) usually do not express cytokeratin AE1/AE3, it is important to remember that 15% of HCCs express AE1/AE3. Likewise, although CK7 expression is not typical of clear cell HCCs, focal positivity does not exclude it.
Take Home Points:
- When a liver biopsy demonstrates a predominantly clear cell neoplasm, a wide differential diagnosis including primary and metastatic clear cell tumors should be considered.
- The presence of cytologic features of classical HCC, bile, or Mallory-Denk bodies within the lesion and/or background chronic liver disease are helpful clues to hepatocellular origin.
- Clear cell HCC may demonstrate patchy and/or weak staining for Hep Par1 and arginase 1. Thus, a panel approach to immunohistochemical work-up (to both rule in and rule out entities) is recommended.
References:
Bansal N, Rastogi M. Clear cell hepatocellular carcinoma with associated tuberculosis-a case report. J Hepatol Gastroint Dis. 2017 Apr; 3:2. doi: 10.4172/2475-3181.1000146.
Christensen WN, Boitnott JK, Kuhajda FP. Immunoperoxidase staining as a diagnostic aid for hepatocellular carcinoma. Mod Pathol. 1989 Jan; 2(1):8-12. https://pubmed.ncbi.nlm.nih.gov/2466290/. Accessed 6/2/20.
Chung YE, Park MS, Park YN, Lee HJ, Seok JY, Yu JS, Kim MJ. Hepatocellular carcinoma variants: radiologic-pathologic correlation. AJR Am J Roentgenol. 2009 Jul; 193 (1):W7-13. doi: 10.2214/AJR.07.3947.
Clayton EF, Furth EE, Ziober A, et al. A case of primary clear cell hepatocellular carcinoma in a non-cirrhotic liver: an immunohistochemical and ultrastructural study. Rare Tumors. 2012 Apr 12;4(2):e29. doi:10.4081/rt.2012.e29
Ko JZH, Chen Y, Nepute J, Goodwill V, Garrett R, Befeler, Lai J. A giant primary clear cell hepatocellular carcinoma without cirrhosis. J Clin Exp Pathol. 2015 Aug; 5:224. doi: 10.4172/2161-0681.1000224.
Li T, Fan J, Qin L, Zhou J, Sun H, Qiu S, Ye Q, Wang L, Tang Z. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011 Jul; 18(7):1955-63. doi: 10.1245/s10434-010-1540-z.
Liu JH, Tsai HL, Hsu SM, Hu DH, Chen SS. Clear cell and non-clear cell hepatocellular carcinoma: a case report and literature review. Kaohsiung J Med Sci. 2004 Feb; 20(2):78-82. doi: 10.1016/S1607-551X(09)70088-9.
Liu Z, Ma W, Li H, Li Q. Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res. 2008 Mar; 38(3):291-9. doi: 10.1111/j.1872-034X.2007.00264.x.
Monzawa S, Omata K, Shimazu N, Yagawa A, Hosoda K, Araki T. Well-differentiated hepatocellular carcinoma: findings of US, CT, and MR imaging. Abdom Imaging. 1999 Jul-Aug; 24(4):392-7. doi: 10.1007/s002619900521.
Mounajjed T, Chandan VS, Torbenson MS (eds). Surgical Pathology of Liver Tumors. Switzerland: Springer International Publishing; 2015.
Murakata LA, Ishak KG, Nzeako UC. Clear cell carcinoma of the liver: a comparative immunohistochemical study with renal clear cell carcinoma. Mod Pathol. 2000 Aug; 13(8):874-81. doi: 10.1038/modpathol.3880156.
Qin J, Higashi T, Nakagawa S, Yamashita, Y-I, Beppu T, Baba H, Kobayashi M, Kumada H, Gunasekaran G, Schiano TD, Thung SN, Fiel MI, Hoshida Y, Ward SC. Steatohepatitic Variant of Hepatocellular Carcinoma Is Associated With Both Alcoholic Steatohepatitis and Nonalcoholic Steatohepatitis: A Study of 2 Cohorts With Molecular Insights. Am J Surg Pathol. 2020. Jun 30. doi: 10.1097/PAS.0000000000001533.
Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012;43(5):737-746. doi:10.1016/j.humpath.2011.07.005
Torbenson M, Zen Y, Yeh MM. Tumors of the liver, AFIP Atlas of tumor pathology series 4. Washington (DC): American Registry of Pathology 2018; 39-112.
WHO Classification of Tumours Editorial Board. Digestive system tumours. Lyon (France): International Agency for Research on Cancer; 2019. (WHO classification of tumours series, 5th ed.; vol. 1). http://publications.iarc.fr/579.
