Interesting Case April 2020
Case contributed by:
Caroline Bsirini MD, Karen Vanderbilt BS, Lee Ann Hellman PA (ASCP),
Michael G. Drage MD, PhD,
University of Rochester Medical Center
Have an interesting case to share?
Contact HPHS newsletter committee chair Sadhna.Dhingra@ProPath.com
Clinical History
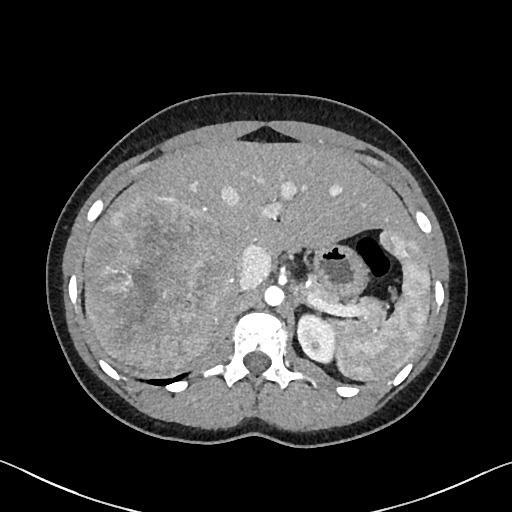
A 15 year old female presented to the emergency department with acute onset of right lower quadrant abdominal pain. A CT scan was performed, which revealed an incidental 14 cm heterogenous mass in the right lobe of the liver, with a central low density stellate scar and a few scattered calcifications. The radiologic differential included focal nodular hyperplasia (favored), hemangioma, fibrolamellar carcinoma, and less likely, hepatoblastoma. The patient’s liver chemistries were within normal limits; AFP was not checked. After biopsy, the patient was referred to our institution for extended right hepatectomy.
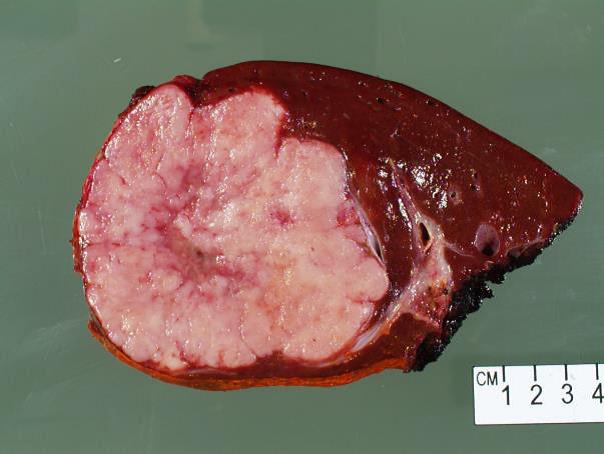
Gross & Microscopic findings:
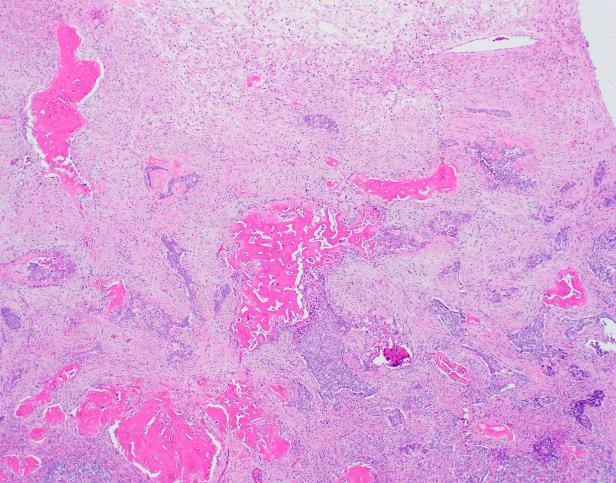
The resection specimen revealed a well-circumscribed tumor with a pale pink parenchyma and a pale tan/brown necrotic/soft stellate area in the center (Fig 1b). The tumor had a gritty cut surface which protruded slightly from the cut surface of the liver. The liver was otherwise unremarkable.
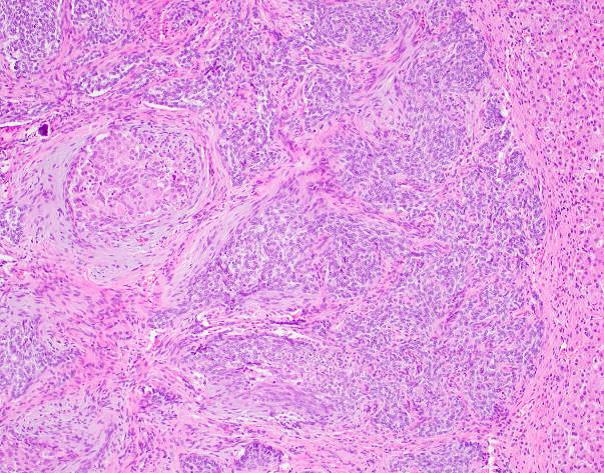
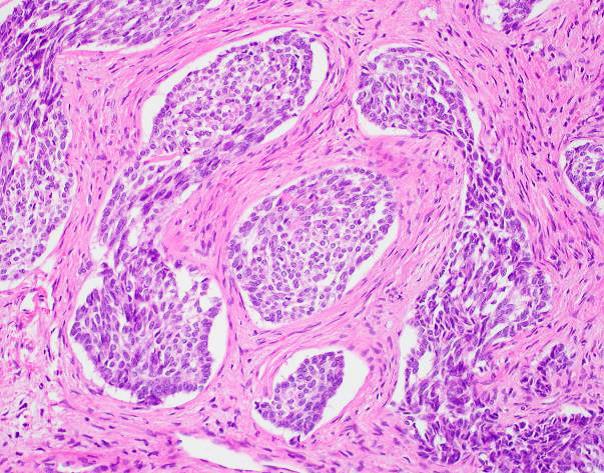
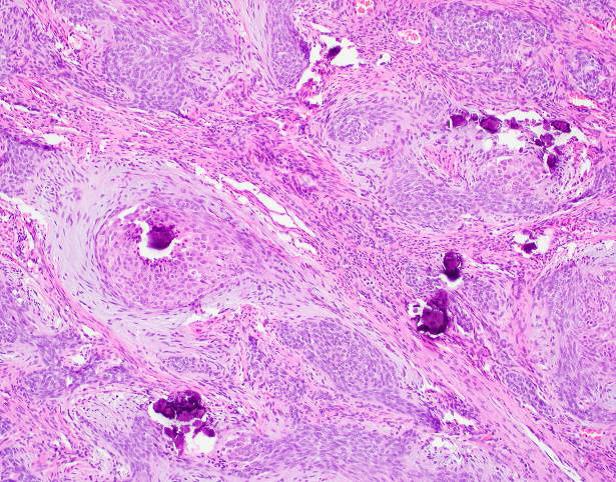
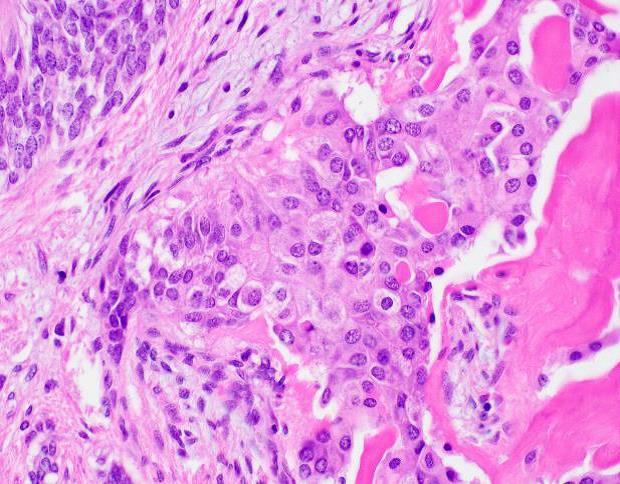
Microscopic examination revealed a well-circumscribed, unencapsulated tumor with a lobulated growth pattern (Fig 2a H&E, original mag 100x). The tumor was distinctly biphasic; with nests of epithelial cells divided by a population of spindled fibroblastic cells (Fig 2b, H&E, original mag 200x). The epithelial cells had variable cytologic features, ranging from medium-sized epithelioid cells with moderate amounts of palely eosinophilic, finely granular cytoplasm and visible cytoplasmic membranes, to small oval-to-spindled cells with minimal cytoplasm and a syncytial appearance (Fig 2c, H&E original magnification 100x). Vague peripheral palisading and retraction artifact were seen in some areas. The stromal component consisted of a uniform fibroblastic proliferation with occasional areas of myxoid matrix condensation around the periphery of some epithelial nests (Fig 2c, H&E original magnification 100x, Fig 2d, H&E original magnification 400x). Coarse calcifications were scattered throughout the tumor, and prominent osseous metaplasia surrounding the central area of degeneration (Fig 2e, H&E original magnification 40x). No perineural or lymphovascular invasion was identified. The background liver parenchyma showed no significant pathologic change.
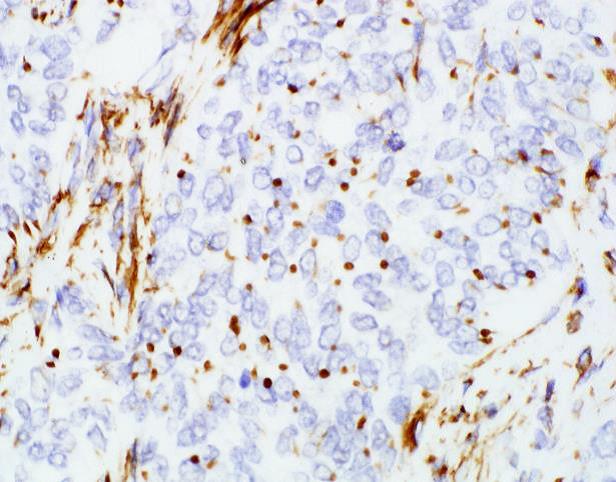
Immunohistochemical stains revealed that the tumor was positive for AE1/AE3, B-catenin (cytoplasmic and nuclear) and WT1 (dot-like perinuclear staining; Fig 2f, original magnification 600x), while negative for ARG-1, HepPar-1 and Desmin.
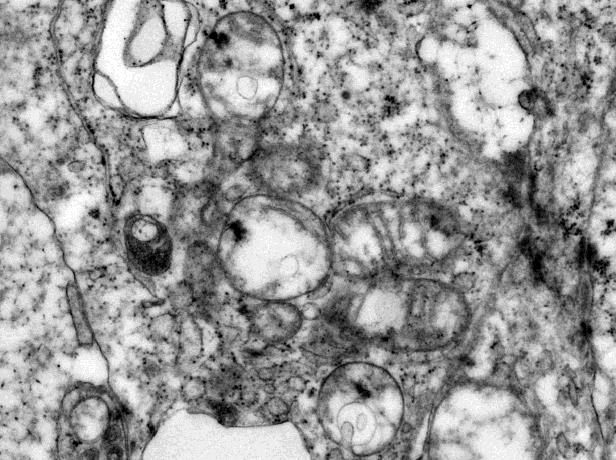
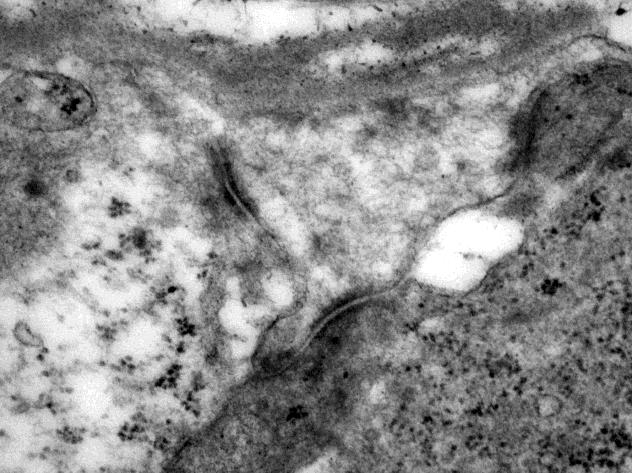
Electron microscopy was performed on glutaraldehyde-fixed tissue. Sections stained with uranyl acetate and lead citrate revealed abundant intermediate filaments and relatively sparse mitochondria with poorly formed and/or poorly preserved cristae in the epithelial cells (Fig 3a, original magnification 20000x). The epithelial cells demonstrated prominent desmosome-like intercellular junctions (Fig 3b, original magnification 40000x).
Diagnosis: Calcifying nested stromal epithelial tumor (CNSET)
Discussion:
Calcifying nested stromal epithelial tumor (CNSET) is a rare primary liver tumor of uncertain histogenesis and low malignant potential (for an excellent review, see Benedict and Xhang [1]). The tumor was first described in 2001 by Ishak et al [2], where it was named “ossifying stromal epithelial tumor”. Other names include “desmoplastic nested spindle cell tumor” [3], “nested stromal epithelial tumor” [4, 5], and “ossifying malignant mixed epithelial and stroma tumor” [6]. These were unified under the rubric of CNSET in 2009 to describe the shared histology of these tumors [7]. To the best of our knowledge, forty-four cases of CNSET have been described in the literature with our case being the 45th [1, 8-13].
CNSETs typically occur in children and young adults (age range 2 to 34 years old; female: male 2:1) [1, 8-13]; one case has been reported in a 77 year old male [10]. Most are large (average size 15.6 cm (range 2.8 to 34cm) [1, 8, 9, 11, 12], located in the right hepatic lobe, and either incidentally discovered or present in the setting of vague abdominal discomfort. A subset of patients present with Cushingoid symptoms secondary to ectopic ACTH production [1, 7]. Liver function tests, AFP and CEA are within normal limits [1, 14]. NSE and CA19-9 have both been reported to be elevated in rare cases [12, 15].
On imaging, CNSETs of the liver are large, heterogeneous, well circumscribed masses with calcifications, hypodense areas representing myxoid or cystic degeneration and, as in this case, occasionally a central scar [16]. Stellate nodules have also been reported [17]. While the radiologic differential diagnosis may include focal nodular hyperplasia and/or calcified hemangioma, in younger patients a solid mass with calcifications may suggest hepatoblastoma, fibrolamellar hepatocellular carcinoma, hamartoma or undifferentiated embryonal sarcoma [18].
On gross examination, CNSETs are pale tan-yellow, well circumscribed, lobulated and commonly display myxoid and calcified areas on cut sections [7]. Microscopically, they consist of haphazardly arranged epithelial islands embedded in a variably dense fibrotic stroma with bile duct proliferation, particularly prominent at the periphery of the lesion. The nested epithelial islands display variable cytological features: spindle, oval, elongated, clear to epithelioid cells [1, 7, 9]. A trabecular architecture resembling well-differentiated neuroendocrine tumor has been reported [4, 7]. The epithelial cells have fine chromatin with indistinct nucleoli. The stromal component is comprised of myofibroblasts. Calcifications and stromal ossifications are consistent findings in most cases. The epithelioid component may show focal rosettes, peripheral palisading, cystic degeneration, and myxoid changes [1]. Most reported cases have low mitotic activity of less than 1 per 10 HPF ; however a few cases have been reported to have up to 10 mitoses per 10 HPF (40x objective) [4, 9], which is associated with a more aggressive clinical course [7]. The background liver parenchyma is typically unremarkable [1, 4, 7].
Immunohistochemistry can be helpful, particularly in limited tissue samples. AE1/AE3 is consistently positive in the epithelial component (at least focally). Nuclear beta catenin is positive in most reported cases. Interestingly WT-1 may show nuclear, cytoplasmic, or dot-like perinuclear staining, the last seen in this case. While of little diagnostic utility, CD56, S100, CD117, EMA, PR, SMA, and vimentin have also been reported positive in some cases. AFP, HepPar-1, inhibin, ER, synaptophysin, chromogranin, desmin, CD99 and CD34 are negative in most of the reported cases [7, 12, 17]. Please refer to table 1. Although not used for routine diagnosis, findings by electron microscopy typically include prominent desmosomes and sparse mitochondria [2].
| IHC Stain | Positivity |
| Cytokeratin (AE1/AE3) Cytokeratin (CAM5.2) CK7 CK19 CK20 CK8 CK18 CK5/6 ACTH Vimentin WT1¹ CD117 CD56 CD99 CD34² Desmin B-catenin SMA in the stroma Chromogranin Synaptophysin S100 HEPPAR-1 Glypican-3 a-Fetoprotein EMA³ Estrogen receptor Progesterone receptor Inhibin FLI-1 | 42/42 (100%) 9/10 (90%) 10/31 (32%) 10/21 (48%) 0/10 (0%) 5/6 (83%) 6/9 (67%) 2/3 (67%) 7/19 (39%) 20/28 (71%) 34/34 (100%) 14/26 (54%) 22/26 (85%) 3/21 (14%) 9/16 (56%) 0/22 7/7 (100%) 20/26 (77%) 0/24 1/22 (5%) 9/26 (35%) 0/27 1/9 (11%) 0/13 11/22 (50%) 0/9 1/11 (9%) 0/10 1/1 (100%) |
Table 1: Immunohistochemistry profile for CNSET, revised from Geramizadeh et al[18] to include more reported cases [8, 11-13] and our case. ¹ WT1(nuclear, dot-like perinuclear or cytoplasmic expression); ² CD34 (focal staining[3, 4, 19] or punctuate cytoplasmic staining[17])-CD34 was not reported in Geramindez et al, reference: [3, 4, 7-9, 11, 17, 19, 20]; ³ EMA: Epithelial membrane antigen.
The histogenesis and molecular pathology of CNSET is poorly characterized. A few cases have been reported to occur in association with Beckwith Wiedemann syndrome, Klinefelter syndrome, fragile X syndrome and Wilms tumor, but there are similar associations with other liver tumors (mesenchymal hamartoma, hepatoblastoma) [4, 7, 12, 16, 21]. A complex karyotype was identified in one CNSET case [17]. EWS and SYT (SS18) gene rearrangements were negative by real time PCR (in ten cases) and FISH (in one case) [3, 7, 13]. A β-catenin mutation (large deletion in exon 3) was identified in the N- terminal domain in two cases, leading to loss of β-catenin degradation and accumulation within the cytoplasm and nuclear translocation (supported by immunohistochemistry). The same group also identified an up-regulation of master genes in mesenchymal transition (SNAIL, SLUG, TWIST), which led the authors to propose a mechanism of defective mesenchymal epithelial transition [22]. A hyperestrogenic state may also play a role in tumorigenesis, supported by cases that arise in the setting of oral contraceptive use and Klinefelter syndrome [12].
Biphasic liver tumors in the pediatric and early adult population include teratoid and mixed epithelial-mesenchymal hepatoblastoma, synovial sarcoma, teratoma, and desmoplastic small round cell tumor, or metastatic Wilm’s tumor. CNSET is morphologically distinct from these entities, but small samples could lead to diagnostic difficulty. The epithelial component of mixed epithelial-mesenchymal variant of hepatoblastoma is either primitive cells (embryonal pattern) and/or a recapitulation of fetal hepatocytes (fetal pattern) which is distinct from the epithelial component seen in CNSET. Hepatoblastomas usually produce AFP and are positive for HepPar-1 (except the small cell variant), neither seen in CNSET. Teratoma or teratoid hepatoblastoma has teratoid elements, which are not present in CNSET. Biphasic synovial sarcomas have glandular epithelial elements and plump spindle cells with the same nuclear features as the epithelial elements, a clear distinguishing feature from CNSET. Wilms tumor is triphasic with blastemal cells and epithelial tubular or rosette-like components which are less mature than the epithelial islands and different morphologically from the bile duct proliferation in CNSET, respectively. The presence of nests or sheets of small primitive round blue cells in desmoplastic stroma as well as the presence of EWS-WT1 or EWS-ERG is helpful to differentiate desmoplastic small round cell tumor from CNSET [7, 14].
Most of the reported patients with CNSET have been treated with partial hepatectomy. Liver transplantation has been performed when complete excision was not possible. A subset of patients in the literature have been treated with neoadjuvant chemotherapy based on the presumed diagnosis of hepatoblastoma. CNSETs have a low recurrence risk, with recurrence after a long latent period (up to 168 months reported) [7, 16]. Large tumor size, infiltrative growth, vascular invasion, necrosis and elevated mitotic activity are reported to be poor prognostic factors that predict recurrence [7, 17]. There are only three reports of metastatic disease; two metastatic to extrahepatic lymph nodes and one to the lungs [5, 12, 17]. A calcified nodule in the liver since childhood has been described in some cases, supporting that these tumors have an indolent course [7]. The utility of chemotherapy is not clear for these tumors [1].
CNSET is an uncommon primary liver neoplasm of uncertain histogenesis and indolent behavior, predominantly in children and young adults. The tumor has a distinctive morphology, and immunohistochemistry can be helpful in the setting of small biopsies. Surgical resection is typically curative. It is important for pathologists and clinicians to be aware of this entity to avoid overly aggressive management.
References
1. Benedict M, Zhang X. Calcifying Nested Stromal-Epithelial Tumor of the Liver: An Update and Literature Review. Arch Pathol Lab Med, 2019; 143(2): p. 264-268.
2. Ishak K, Goodman ZD, Stocker JT. Miscellaneous malignant tumors. In:Tumors of the Liver and Intrahepatic Bile Ducts, 2001, Washington, DC: Armed Forces Institute of Pathology. p. 271–278.
3. Hill D, Swanson P, Anderson K, Covinsky M, Finn L, Ruchelli E et al. Desmoplastic nested spindle cell tumor of liver: report of four cases of a proposed new entity. Am J Surg Pathol, 2005; 29(1): p. 1-9.
4. Heerema-McKenney A, Leuschner I, Smith N, Sennesh J, Finegold M. Nested stromal epithelial tumor of the liver: six cases of a distinctive pediatric neoplasm with frequent calcifications and association with cushing syndrome. Am J Surg Pathol, 2005; 29(1): p. 10-20.
5. Hommann M, Kaemmerer D, Daffner W, Prasad V, Baum R, Petrovitch A et al. Nested stromal epithelial tumor of the liver–liver transplantation and follow-up. J Gastrointest Cancer, 2011; 42(4): p. 292-5.
6. Heywood G, Burgart L, Nagorney D. Ossifying malignant mixed epithelial and stromal tumor of the liver: a case report of a previously undescribed tumor. Cancer, 2002; 94(4): p. 1018-22.
7. Makhlouf H, Abdul-Al H, Wang G, Goodman Z. Calcifying nested stromal-epithelial tumors of the liver: a clinicopathologic, immunohistochemical, and molecular genetic study of 9 cases with a long-term follow-up. Am J Surg Pathol, 2009; 33(7): p. 976-83.
8. Ianole V, Ungureanu L, Morosan E, Lupascu C. Calcifying nested-stromal epithelial tumor (CNSET) of the liver. Archive of clinical cases 2018; 5(1).
9. Meletani T, Cantini L, Lanese A, Nicolini D, Cimadamore A, Agostini A et al. Are liver nested stromal epithelial tumors always low aggressive? World J Gastroenterol, 2017; 23(46): p. 8248-8255.
10. Sara H, Kara T, Ates F, Coban M, Durmaz M, Gunler T. Presentation of an Extremely Rare Tumor: Nested Stromal-epithelial Tumor of the Liver. EJMO 2019; 3(1):p. 66-69.
11. Taylor K. Calcifying Nested Stromal-Epithelial Tumor of the Liver: A Case Report and Review of Literature. The journal of american association of pathologists’ assistants, 2015;5: p. 5.
12. Tsuruta S, Kimura N, Ishido K, Kudo D, Sato K, Endo T et al. Calcifying nested stromal epithelial tumor of the liver in a patient with Klinefelter syndrome: a case report and review of the literature. World J Surg Oncol, 2018;16(1): p. 227.
13. Lai Z, Li W, Aqel Z, Macknis J. Synaptophysin-positive nested stromal epithelial tumor (NSET) of the liver in a 13 year old male: A case report. Hum Pathol 2019;16.
14. Misra S and Bihari C. Desmoplastic nested spindle cell tumours and nested stromal epithelial tumours of the liver. APMIS, 2016; 124(4): p. 245-51.
15. Procopio F, Di Tommaso L, Armenia S, Quagliuolo V, Roncalli M, Torzilli G. Nested stromal-epithelial tumour of the liver: An unusual liver entity. World J Hepatol, 2014; 6(3): p. 155-9.
16. Schaffer L, Shehata B, Yin J, Schemankewitz E, Alazraki A. Calcifying nested stromal-epithelial tumor (CNSET) of the liver: a newly recognized entity to be considered in the radiologist’s differential diagnosis. Clin Imaging, 2016; 40(1): p. 137-9.
17. Brodsky S, Sandoval C, Sharma N, Yusuf Y, Facciuto M, Humphrey M. Recurrent nested stromal epithelial tumor of the liver with extrahepatic metastasis: case report and review of literature. Pediatr Dev Pathol, 2008; 11(6): p. 469-73.
18. Geramizadeh B. Nested Stromal-Epithelial Tumor of the Liver: A Review. Gastrointestinal Tumors, 2019; 6(1-2): p. 1-10.
19. Oviedo Ramirez M, Bas Bernal A, Ortiz Ruiz E, Bermejo J, De Alava E, Hernandez T. Desmoplastic nested spindle cell tumor of the liver in an adult. Ann Diagn Pathol, 2010; 14(1): p. 44-9.
20. Wang Y, Zhou J, Huang W, Rao Q, Ma H, Zhou X. Calcifying nested stroma-epithelial tumor of the liver: a case report and review of literature. Int J Surg Pathol, 2011; 19(2): p. 268-72.
21. Wirojanan J, Kraff J, Hawkins D, Laird C, Gane L, Angkustsiri K et al. Two boys with fragile x syndrome and hepatic tumors. J Pediatr Hematol Oncol, 2008; 30(3): p. 239-41.
22. Assmann G, Kappler R, Zeindl-Eberhart E, Schmid I, Haberle B, Graeb C et al. beta-Catenin mutations in 2 nested stromal epithelial tumors of the liver–a neoplasia with defective mesenchymal-epithelial transition. Hum Pathol, 2012; 43(11): p. 1815-27.
