Interesting Case July 2019
Case contributed by:
Regina Kwon, MD, MPH, and Kiyoko Oshima, MD, PhD
Johns Hopkins University School of Medicine
Clinical history
A 62-year-old man, recently diagnosed with cirrhosis due to alcohol use and previously unknown hepatitis C infection, was referred to our institution for transplant evaluation. CT imaging demonstrated a markedly nodular liver with scattered hypodense lesions up to 1 cm, considered likely to be cysts. A follow-up MRI identified a 2.2 cm nodule suggestive of either dysplasia or regeneration. Alpha-fetoprotein was 160 ng/mL. Three months later, the patient was admitted for acute kidney injury, hyponatremia, and hyperkalemia. His MELD-Na score was 34 and alpha-fetoprotein 47 ng/mL. His prognosis was considered poor. Soon thereafter, the patient underwent increased-risk deceased-donor liver transplant.
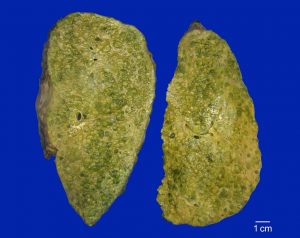
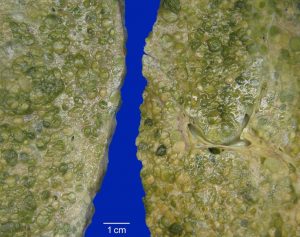
The explanted liver weighed 2,410 g and was diffusely nodular. Two regenerative nodules, measuring 0.6 cm and 2.3 cm, were identified.
Liver explant microscopic findings
The explant showed moderately differentiated hepatocellular carcinoma (HCC) involving more than 95% of the hepatic parenchyma, including the regenerative nodules identified during gross examination. A representative section was taken from each segment and all segments showed HCC. The average tumor nodule size was 1 cm. Trabecular (nested) and pseudoglandular growth patterns were present. There was lymph-vascular space invasion, but large vessels were spared. The background liver showed cirrhosis, steatosis, chronic hepatitis, and mild iron overload (reticulin, iron, and Masson trichrome stains).
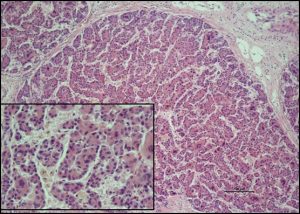 |
| H&E stain: Trabecular architecture (x10) of the tumor. Inset x20 |
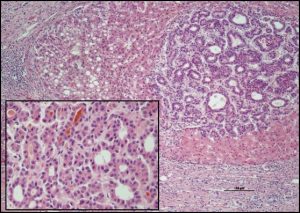 |
|
H&E stain: Pseudoglandular architecture (x10) of the tumor. Inset x20.
|
 |
|
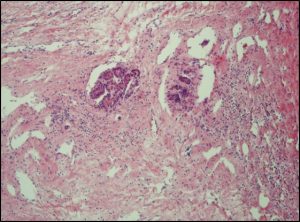
H&E stain: Lymph-vascular space invasion (x10)
|
Diagnosis
Cirrhotomimetic hepatocellular carcinoma (CM HCC).
Discussion
Cirrhotomimetic hepatocellular carcinoma is relatively rare. Frequencies of up to 10% in autopsy specimens have been described, and one recent single-institution series reported an incidence of 13% over an eight-year period in patients with HCC. No specific risk factors for developing CM HCC have been identified beyond those known for developing HCC in general, although some studies suggest associations with younger age and hepatitis B infection.
The infiltrative pattern and lack of distinct borders of CM HCC make it difficult to detect by imaging. Moreover, since cirrhosis is nearly always present, the tumor is often indistinguishable from the heterogeneity of cirrhosis on imaging. The lack of consideration of this entity can have serious clinical consequences due not only to delays in growth treatment but also to the misallocation of donor livers.
Of all types of HCC, only the cirrhotomimetic type is defined by the gross findings. On examination, CM HCC is diffusely nodular, with nodules that cannot be readily differentiated from those of cirrhosis. Thin sections are necessary to rule out a conventional HCC that has a dominant nodule with satellite nodules, as this may be confused with CM HCC, particularly on imaging studies. Indeed, although CM HCC nodules may coalesce into a mass-like lesion, mimicking a dominant nodule, the two entities can be distinguished by the number of nodules: virtually all conventional dominant nodules have fewer than 10 satellite nodules, while in CM HCC, there are nearly always more than 30 satellites. Finally, because CM HCC may be geographically restricted within a broadly cirrhotic liver, it is important to identify and sample any nodules that differ in size or quality from the majority of nodules.
Microscopically, CM HCC consists of tumor nodules scattered throughout the hepatic parenchyma; when background cirrhosis is present, as is typically the case, the tumor is described as “interdigitating” among the cirrhotic nodules. However, this is an unreliable criterion, as conventional HCC may also show interdigitation. CM HCC is otherwise similar to traditional HCC and assumes one of the known patterns, such as solid, pseudoglandular, trabecular, and clear cell.
CM HCC is generally believed to have a poor prognosis, although this may not be true for all subtypes. For example, in a series of 26 CM HCCs, Clayton et al found that patients whose tumors had clear cell morphology and were “confined” (involving < 50% of the liver) experienced markedly better 5-year survival (67% vs. 13%) than those not meeting these criteria. In our case of an extensive CM HCC (involving > 50% of the liver) with pseudoglandular and trabecular morphologies, the patient experienced a recurrence in his transplanted liver within one year and bony metastases within 18 months.
The infiltrative pattern and lack of distinct borders of CM HCC make it difficult to detect by imaging. Moreover, since cirrhosis is nearly always present, the tumor is often indistinguishable from the heterogeneity of cirrhosis on imaging. The lack of consideration of this entity can have serious clinical consequences due not only to delays in growth treatment but also to the misallocation of donor livers.
Of all types of HCC, only the cirrhotomimetic type is defined by the gross findings. On examination, CM HCC is diffusely nodular, with nodules that cannot be readily differentiated from those of cirrhosis. Thin sections are necessary to rule out a conventional HCC that has a dominant nodule with satellite nodules, as this may be confused with CM HCC, particularly on imaging studies. Indeed, although CM HCC nodules may coalesce into a mass-like lesion, mimicking a dominant nodule, the two entities can be distinguished by the number of nodules: virtually all conventional dominant nodules have fewer than 10 satellite nodules, while in CM HCC, there are nearly always more than 30 satellites. Finally, because CM HCC may be geographically restricted within a broadly cirrhotic liver, it is important to identify and sample any nodules that differ in size or quality from the majority of nodules.
Microscopically, CM HCC consists of tumor nodules scattered throughout the hepatic parenchyma; when background cirrhosis is present, as is typically the case, the tumor is described as “interdigitating” among the cirrhotic nodules. However, this is an unreliable criterion, as conventional HCC may also show interdigitation. CM HCC is otherwise similar to traditional HCC and assumes one of the known patterns, such as solid, pseudoglandular, trabecular, and clear cell.
CM HCC is generally believed to have a poor prognosis, although this may not be true for all subtypes. For example, in a series of 26 CM HCCs, Clayton et al found that patients whose tumors had clear cell morphology and were “confined” (involving < 50% of the liver) experienced markedly better 5-year survival (67% vs. 13%) than those not meeting these criteria. In our case of an extensive CM HCC (involving > 50% of the liver) with pseudoglandular and trabecular morphologies, the patient experienced a recurrence in his transplanted liver within one year and bony metastases within 18 months.
Learning points
1. Cirrhotomimetic carcinoma is a rare aggressive subtype of hepatocellular carcinoma characterized by numerous small tumor nodules throughout the liver.
2. This entity should not be confused with a dominant hepatocellular carcinoma with multiple satellite nodules. The gross examination is critical to accurate diagnosis.
3. Imaging has poor sensitivity for detection of this entity.
Acknowledgments
The specimen was grossed by Dr. Lais Osmani.
Photography assistance by Norm Barker and George Lopez.
References
1. Agni RM. Diagnostic histopathology of hepatocellular carcinoma: a case-based review . Semin Diagn Pathol. 2017;34(2):126-37.
2. Clayton EF et al. Liver transplantation and cirrhotomimetic hepatocellular carcinoma: classification and outcomes. Liver Transpl. 2014;20(7):765-74.
3. Kanematsu M, Semelka RC, Leonardou P, Mastropasqua M, Lee JK. Hepatocellular carcinoma of diffuse type: MR imaging findings and clinical manifestations. J Magn Reson Imaging. 2003;18(2):189-95.
4. Torbenson MS. Hepatocellular carcinoma. In: Torbenson MS, Zhang L, Moreira RK, eds. Surgical Pathology of the Liver. 1st ed. Philadelphia, PA:Wolters-Kluwer; 2018. pp. 598-650.