Liver Biopsy Findings:
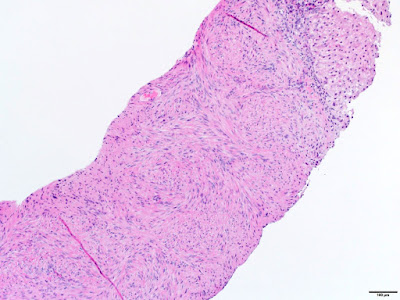
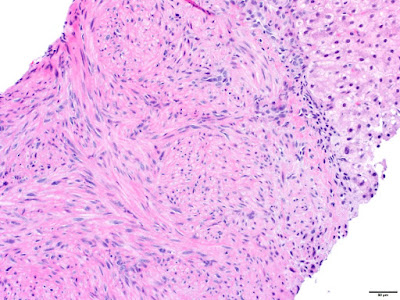
The liver biopsy showed spindled tumor cells arranged in short intersecting fascicles. The tumor cells were bland, demonstrating elongated nuclei with blunted ends and abundant eosinophilic cytoplasm. No significant cytologic atypia was identified. Mitotic activity and tumor necrosis were not conspicuous. The background liver was unremarkable.
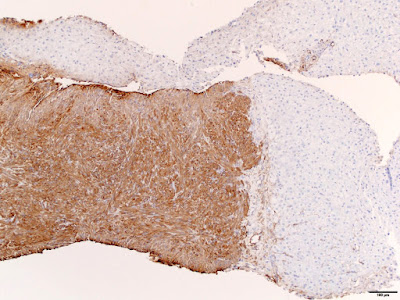
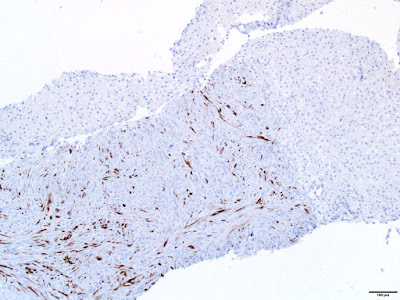
Immunohistochemical stains demonstrated that tumor cells were diffusely positive for smooth muscle actin (SMA) and patchy positive for desmin. In situ hybridization for Epstein-Barr virus encoded RNA (EBER) was strongly and diffusely positive in the tumor cells.
Diagnosis:
EBV-associated smooth muscle tumor
Discussion:
Epstein-Barr virus (EBV)-associated smooth muscle tumor is a rare neoplasm that is most commonly seen in immunocompromised patients, including those with organ transplants, HIV/AIDS, and congenital immunodeficiency1. The lesions can involve a variety of anatomic sites and multifocal tumors are thought to be due to multiple infections rather than metastatic disease2. In post-transplant recipients, the most common locations of tumor occurrence are the liver, lungs, larynx, pharynx, gastrointestinal tract, spleen, kidneys and central nervous system. Histopathologic features may vary in terms of cellular atypia, mitotic activity, and necrosis, however unlike conventional smooth muscle tumors, these features do not correlate well with tumor behavior.
Local surgical resection, reduced immunosuppression, and chemotherapeutic approaches have been used in the treatment of these tumors. Most patients succumb to infectious complications or underlying disease rather than tumor progression, however HIV associated tumors and tumors of the CNS have been reported to have the worst prognoses.
Histologic features
The histologic features of these tumors include spindled cells arranged in fascicles. Tumor cells have eosinophilic cytoplasm with elongated, blunt-ended nuclei. Two unique histologic features have been described, which include a variable number of intratumoral lymphocytes and occasional primitive round cell areas. Histopathologic features may vary in terms of cellular atypia, mitotic activity, and necrosis.
Ancillary testing
The critical ancillary diagnostic finding is positive in situ hybridization for EBER. The tumor cells are positive for SMA and may be positive for desmin. They are negative for S100, HMB45, CD34, CD117, and HHV-8.
Differential diagnosis
These tumors demonstrate a relatively bland spindle cell proliferation, and the morphologic differential diagnosis includes mesenchymal tumors such as solitary fibrous tumor, leiomyoma, schwannoma, neurofibroma, angiomyolipoma, gastrointestinal stromal tumor, and inflammatory myofibroblastic tumor3. Due to the patient’s clinical history of post-organ transplant immunosuppression, Kaposi sarcoma and mycobacterial spindle cell pseudotumor may also be considered. In this case, the tumor cells were diffusely and strongly positive for EBER and SMA, which confirmed the diagnosis.
In conclusion, EBV-associated smooth muscle tumor is a rare lesion that can occur in the liver of immunocompromised patients. In situ hybridization for EBER confirms the diagnosis. The patient has undergone partial left liver resection of the lesion and is currently doing well. His hyperbilirubinemia resolved without elucidation of a definitive etiology, but it was thought not to be related to the tumor.
References:
1. Dekate J, Chetty R. Epstein-Barr virus-associated smooth muscle tumor. Arch Pathol Lab Med. 2016;140(7):718-22.
2. Deyrup AT, Lee VK, Hill CE, et al. Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients. Am J Surg Pathol. 2006;30(1):75-82.
3. Burt A, Portman B, Ferrell L. MacSween’s Pathology of the Liver. 6th ed. Edinburgh: Churchill Livingston Elsevier; 2012. Chapter 14, Tumours and tumour-like lesions of the liver; p. 818-819.
This case was submitted by:
Katherine E. Boylan, MD, Resident, Department of Pathology, University of Utah, Salt Lake City, UT
Mazdak Khalighi, MD, Assistant Professor, Department of Pathology, University of Utah, Salt Lake City, UT
Eric Swanson, MD, Assistant Professor, Department of Pathology, University of Utah, Salt Lake City, UT