Interesting Case May 2024
Case contributed by:
Harjot Gill, MD, PGY-1, Montefiore Medical Center, The University Hospital for Albert Einstein College of Medicine, Bronx, New York, USA
Qiang Liu, MD, PhD, Professor, Montefiore Medical Center, The University Hospital for Albert Einstein College of Medicine, Bronx, New York, USA
Yanan Fang, MD, PhD, Associate Professor, Montefiore Medical Center, The University Hospital for Albert Einstein College of Medicine, Bronx, New York, USA
Jay H Lefkowitch, MD, Professor, New York Presbyterian/ Columbia University Irving Medical center, New York, NY, USA
Amarpreet Bhalla, MD, Associate Professor, Montefiore Medical Center, The University Hospital for Albert Einstein College of Medicine, Bronx, New York, USA
Case History
A 55-year-old female presented with alcoholic liver cirrhosis, ascites, hepatic hydrothorax, and
portosystemic encephalopathy. She underwent living donor liver transplant from her son. A liver biopsy
taken two weeks after transplant surgery revealed mild acute cellular rejection and moderate
preservation injury. Her post-operative course was complicated by small for size syndrome,
intramuscular hematoma with VRE bacteremia, biliary stricture, and inferior vena cava stenosis. The
biliary stricture and inferior vena cava stenosis were stented three months post-transplant. Concomitant
liver biopsy showed late allograft rejection with perivenular plasma cell infiltrates and mild centrilobular
fibrosis. The plasma cells were polyclonal with an unremarkable kappa lambda ratio. C4d immunostain
was negative. She was treated with steroids and her liver enzymes improved.
Four months later, she presented with subtherapeutic Prograf (Tacrolimus) levels and a cholestatic
pattern of elevated liver function tests. ERCP revealed an intact stent within the stricture and a
gallstone, both of which were removed. Her liver function tests continued to worsen with no detectable
abnormality on repeat ERCP but improvement with antibiotics. Hematologic evaluation revealed an
abnormal serum protein electrophoresis (SPEP). Monoclonal IgG kappa was elevated along with a mildly
elevated free kappa/lambda ratio. The patient deferred bone marrow biopsy. Two months later (nine
months post-transplant), she was re-admitted for worsening liver function tests and another liver biopsy
was performed.
Pathology Findings
The biopsy revealed a moderate centrilobular, sinusoidal and portal/periportal inflammatory infiltrate
predominantly comprised of plasma cells. There was mild interface and lobular activity, centrilobular
hepatocyte dropout, clusters of ceroid-laden Kupffer cells and bile duct injury. Patchy, minimal portal
vein endotheliitis was present (Fig. 1). IgG4 highlighted up to 35 plasma cells per high power field at hot
spots in portal tracts (Fig. 2, Fig. 3). The IgG4/IgG ratio was approximately 30%. Immunohistochemistry
showed an atypical kappa predominance of plasma cells (Fig. 4). CD3 and CD20 highlighted a few T and B
cells respectively. Given the increased IgG on SPEP, high kappa/lambda ratio, and a serum M-spike, the
hepatic infiltrate was consistent with a Kappa and IgG4 restricted plasma cell neoplasm. Epstein Barr
virus early antigen (EBNA) and in-situ hybridization tests (EBER ISH), herpes simplex virus and
cytomegalovirus DNA PCR were negative. The patient was treated with a steroid pulse and her liver
function tests improved.

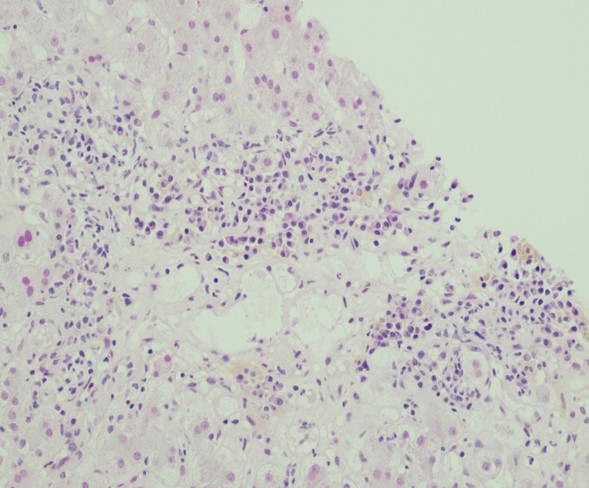

Figure 1a, b, c. H&E, 200x, 400x: Liver core biopsy demonstrates a moderate centrilobular, sinusoidal and portal/periportal inflammatory infiltrate predominantly composed of plasma cells.

Figure 2. CD138, 400x: Immunohistochemical stain for CD138 highlights plasma cells.

Figure 3. IgG4, 400x: IgG4 immunostain highlights approximately 35 plasma cells per high power field focally.

Figure 4. Kappa, 400x: Kappa immunostain demonstrates kappa predominant plasma cells (kappa to lambda ratio of 8–10:1).

Figure 5. CD3, 400x
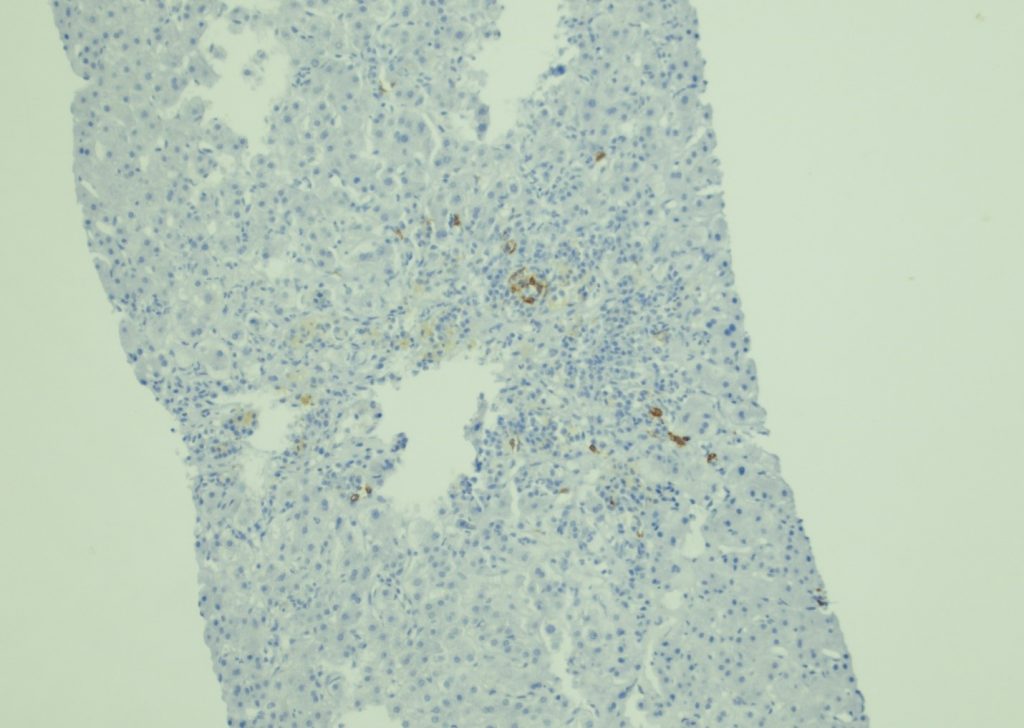
Figure 6. CD20, 400x

Figure 7. EBER in-situ hybridization, 400x
Diagnosis
Kappa and IgG4 restricted plasma cell neoplasm
Follow-Up
The Hematology oncology team followed up on this case. The differential diagnosis of a hepatic clonal plasma cell infiltrate was either an extramedullary myeloma, post-transplant lymphoproliferative disorder (PTLD)/lymphoid proliferation or lymphoma associated with immune deficiency and dysregulation (IDD-LPDs). A negative Epstein Barr virus in-situ hybridization test excluded PTLD. The hematological evaluation revealed pancytopenia and a Pelger Huet abnormality. Skeletal survey did not show destructive lytic or blastic lesions. She was followed up regularly. Her paraprotein levels continued to slowly rise, nearly doubling every two months. Her treatment plan included carfilzomib based induction therapy and dexamethasone to prevent end-organ damage from evolving myeloma. She was also considered for stem cell transplant. In one of her follow up visits, the patient presented with acute appendicitis and an appendectomy was performed. The patient passed away during her post-operative course.
Discussion
This is the first documented case of IgG4 plasma cell dyscrasia in a liver transplant biopsy in the medical literature. The histological features overlap with late allograft rejection. However, the plasma cell infiltrate far outweighed the lymphocytes, a distinct contrast from typical acute cellular rejection. Also, the corresponding SPEP was abnormal, prompting evaluation for clonality of plasma cells. Immunohistochemistry confirmed the kappa restricted nature of the plasma cells, in contrast to a polyclonal plasma cell population in a previously performed biopsy with late allograft rejection. Clonality analysis for Immunoglobulin heavy and light chain genes rearrangement supported the diagnosis.
Solitary extramedullary plasmacytoma of the liver has been previously reported, although the IgG isotype was not analyzed in those cases [6], [7], [8], [9], [10], [11], [12]. A few published cases were associated with systemic monoclonal gammopathy. However, liver involvement is rare and usually not the first presentation [10]. In prior studies, plasma cell neoplasms presenting in the liver showed an association with a plasmablastic morphology. However, this present case did not demonstrate plasmablastic cells [1]. The pathogenesis of a plasma cell neoplasm in the transplant setting is debatable. In this case, the neoplastic clone may have evolved from a plasma cell infiltrate, initially presenting as plasma cell rich rejection, which is known to be comprised of a large proportion of IgG4 positive plasma cells [13]. The cytokine and hormonal milieu in the transplant setting along with existing comorbidities may contribute to clonal evolution of the disease. The complex immunological interactions of donor organ and immunocompromised recipient may also play an important role. On the other hand, the biopsy may represent two independent pathologic processes where the liver is colonized and involved by a malignant clone arising in the bone marrow. Since the bone marrow was not evaluated, the distinction of primary extramedullary hepatic plasmacytoma or myeloma with subsequent extramedullary hepatic involvement cannot be made.
IgG4 plasma cell myeloma cases comprised 4% of all myeloma cases in a 2013 study. The prevalence correlated with a normal distribution of IgG4 isoform. The clinical course is characterized by disease relapses and exacerbations with fluctuating levels of paraproteins. Correlation with necrotizing fasciitis has been reported where bone marrow infiltrates show increased plasma cells but no histologic features pathognomonic for IgG4 related disease [1]. The single case of IgG4 myeloma presenting as a thyroid mass had concomitant involvement of the bone marrow and increased serum levels of IgG43. The unique biochemical structure of IgG4 limits its ability to form immune complexes or to activate the complement cascade. It also inhibits mast cell degranulation. Consequently, it has a protective role for inflammatory and allergic conditions but a deleterious role in anti-tumor responses. In IgG4-related disease, serum levels of IgG4 are raised and IgG4 positive polyclonal plasma cell infiltrates are present in tissues. IgG4 positive polyclonal plasma B cell infiltrates are otherwise reported in extrahepatic cholangiocarcinoma, pancreatic carcinoma and malignant melanoma [2,6,14]
Learning Points
- Plasma cell rich immune infiltrates in allograft liver are mostly comprised of polyclonal cells; rarely a monoclonal plasma cell neoplasm may be present.
- A plasma cell neoplasm can present in the transplant setting as a distinct entity unrelated to EBV associated post-transplant lymphoproliferative disorder (PTLD).
- IgG4 plasma cell neoplasms are not associated with systemic fibroinflammatory IgG4 related disease.
References
1. Geyer J.T., Niesvizky Ruben, Jayabalan David S, et al. IgG4 plasma cell myeloma: new insights into the pathogenesis of IgG4-related disease. Mod. Pathol. 2014;2:375–381. [PubMed] [Google Scholar]
2. Chen Luke Y.C., Mattman Andre, Seidman Michael A., Carruthers Mollie N. IgG4-related disease: what a hematologist needs to know. Haematologica. 2019;104(3):444–455. [PMC free article] [PubMed] [Google Scholar]
3. Funada Masashi, Nakano Kazuhisa, Miyata Hiroko, Nawata Aya, Tanak Yoshiya. IgG4-type multiple myeloma with diffuse enlargement of the thyroid requiring differentiation from IgG4-related disease. Intern. Med. 2020;59:711–714. [PMC free article] [PubMed] [Google Scholar]
4. Ito A., Yamauchi T., Nakano A., Fujino M., Ito M. IgG4 plasma cell myeloma: clinicopathological characteristics and diagnosis. Pathol. Int. 2020;70(8):551–556. Aug. [PubMed] [Google Scholar]
5. Gauiran D.T.V., Marcon K.M., DeMarco M.L., Fung A.W.S., van der Gugten G., Mattman A., Carruthers M.N., Song K.W., Chen L.Y.C. IgG4 plasma cell myeloma without clinical evidence of IgG4-related disease: a report of two cases. Hematology. 2020;25(1):335–340. Dec. [PubMed] [Google Scholar]
6. Kato S., Kuwatani M., Kawakubo K., Sugiura R., Hirata K., Tanikawa S., Mitsuhash T., Shiratori S., Sakamoto N. Hepatobiliary and Pancreatic: pancreatic cancer with elevated serum IgG4 level due to multiple myeloma mimicking localized autoimmune pancreatitis. J. Gastroenterol. Hepatol. 2018;33:1310. [PubMed] [Google Scholar]
7. Lee Jun-Young, Won Jong-Ho, Kim Hyun-Jung, Bae Sang-Byung, Kim Chan-Kyu, Kim Jung Hoon, Lee Nam-Su, Lee Kyu-Taeg, Park Sung-Kyu, Jin So-Young, Hong Dae-Sik, Park Hee-Sook. Solitary extramedullary plasmacytoma of the liver without systemic monoclonal gammopathy. J. Korean Med. Sci. 2007;(4):754–757. Aug; 22. [PMC free article] [PubMed] [Google Scholar]
8. Dohy H., Abe T., Takata N., Fujimura K., Taketomi Y., Kuramoto A., Harada T., Hattori T., Enzan H. Successful hepatectomy for solitary plasmacytoma. N. Engl. J. Med. 1979;300:1218–1219. [PubMed] [Google Scholar]
9. Weichhold W., Labouyrie E., Merlio J.P., Masson B., Mascarel A. Primary extramedullary plasmacytoma of the liver. A case report. Am. J. Surg. Pathol. 1995;19:1197–1202. [PubMed] [Google Scholar]
10. Petrucci M.T., Tirindelli M.C., De Muro M., Martini V., Levi A., Mandelli F. Extramedullary liver plasmacytoma a rare presentation. Leuk. Lymphoma. 2003;44:1075–1076. [PubMed] [Google Scholar]
11. Hyun D.W., Park S.W., Baik J.H., Kim D.H., Jung J.T., Shin D.G., Sohn S.K., Lee K.B. A case of multiple myeloma with multiple intrahepatic extramedullary plasmacytomas. Korean J. Hematol. 1999;34:143–147. [Google Scholar]
12. Chen H.F., Wu T.Q., Li Z.Y., et al. Extramedullary plasmacytoma in the presence of multiple myeloma: clinical correlates and prognostic relevance. Onco Targets Ther. 2012;5:329–334. doi: 10.2147/ott.s35348. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
13. Demetris A.J., Bellamy C., Hubscher S.G., et al. 2016 comprehensive update on the Banff working group on liver allograft pathology: introduction of antibody mediated rejection. Am. J. Transpl. 2016;16:2816–2835. [PubMed] [Google Scholar]
14. Davies Brian Anna M., Sutton J. Human IgG4: a structural perspective. Immunol. Rev. 2015;268:139–159. [PMC free article] [PubMed] [Google Scholar]
Disclosure: The above case has been published in an Elsevier Journal and is available in Pubmed.
Bhalla A, Liu Q, Fang Y, Lefkowitch JH. IgG4 plasma cell neoplasm in liver transplant biopsy masquerading as rejection. Leuk Res Rep. 2023 Jul 8;20:100379. doi: 10.1016/j.lrr.2023.100379. PMID: 37521581; PMCID: PMC10371806.
